Slaked lime ((CaOH)2) also known as Calcium hydroxide, is a white, powdery substance that produced a reaction between calcium oxide (also known as quicklime) and water.
Slaked lime is also known as caustic lime, hydrated lime, slack lime, and pickling lime.
| Technical Name of compound | Slaked lime is also known as Calcium hydroxide Ca(OH)2 | |
| Other names | caustic lime, hydrated lime, slack lime, and pickling lime | |
| Appearance | White powder | |
| Melting point | 853 Kelvin | |
| Molar mass | 74.1 grams per mole | |
| Density | 2.211 grams per cubic centimeter | |
| Uses | Used in the preparation of a dry mixer for painting and whitewashing. |
The most frequent technique for producing slaked lime is the reaction of calcium oxide (commonly known as quicklime) with water. When mixed with water, only a tiny amount of the quicklime dissolves, resulting in the creation of limewater. The rest of the quicklime is left in a suspension, which is commonly referred to as lime milk.
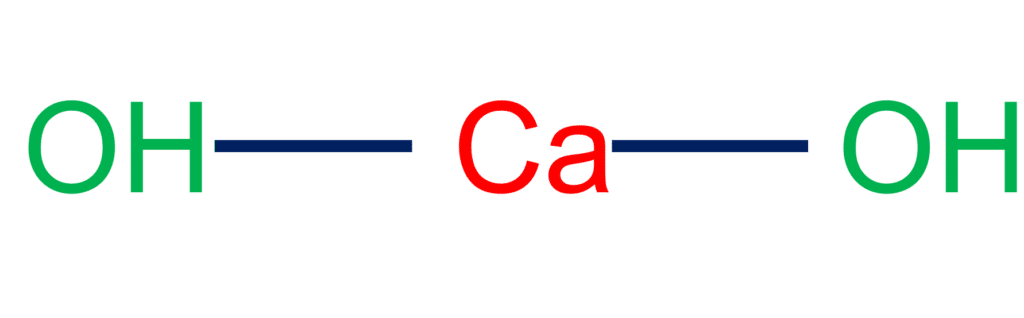
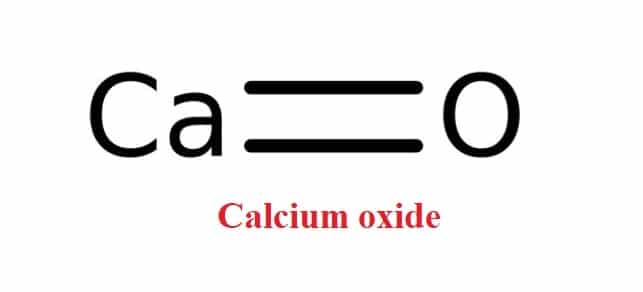
In addition, Calcium oxide can be produced in a lime kiln by thermal decomposition of materials containing calcium carbonates (CaCO3; mineral calcite), such as limestone or seashells. Calcination is the process by which burnt lime is produced.
CaCO3 → CaO + CO2
CaO +H2O → Ca(OH)2
The temperature range for calcination of calcium carbonate is 1070-127 oC. Typically, these reactions occur in a rotary kiln, and the products and results of those reactions are carbon dioxide and burnt lime. Following Le-principle, Chatelier’s the formed carbon dioxide is removed instantly, allowing the reaction to be preceded until the process is completed.
Table of Contents
Element Calcium
Calcium (Ca) is the fifth element and the third most abundant metal in the earth’s crust, with an atomic number of 20. It is a trimorphic metal that is tougher than sodium but softer than aluminum. It has the same qualities as beryllium and aluminum, however, unlike alkaline metals, it does not produce skin burns. It has a lower chemical reactivity than alkaline metals and alkaline-earth metals.
Summary
- Slaked lime, also known as calcium hydroxide, is a white powdery material formed by the reaction of calcium oxide (commonly known as quicklime) with water.
- The most common method for the preparation of slaked lime is via the reaction between calcium oxide (also known as quicklime) and water
Related Links
Soft Water vs. Hard Water
The pH of Distilled/ De-Ionized Water
Acidic Hydrogen
Is Chlorine a metal?| A Simple Overview
Liquid Oxygen-Cryogenic Liquid
Frequently Asked Questions
1. What are ionic compounds?
Ionic compounds are formed by a process known as electron transfer, in which one atom transfers electrons to another. An atom of one element loses one or more electrons during electron transfer, and an atom of another element gains those electrons. Both atoms are involved in the electron transfer from ions.
2. What is the process of distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
3. What is the combustion process?
Combustion is a chemical process in which heat and light are produced. The most common sort of combustion is fire.
4. Is zinc a strong metal?
Zinc metal is a low-tensile-strength metal with less than half the tensile strength of mild carbon steel.
More Links
Molar Mass of Acetic Acid| Easy-Explanation
How Much is a Liter of Water?| Glasses, Bottles, Cups, and
How Many Fluid Ounces Equal a Gallon
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
Liquid Oxygen-Cryogenic Liquid
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023