Silicic acid (H4SiO4) is a compound of silicon, oxygen, and hydrogen and is thought to be the parent material from which a broad family of minerals, salts, and esters (the silicates) are produced. As shown in the figure below the silicic acid molecule contains a total of 8 bond(s). There are 4 non-H bond(s) and 4 hydroxyl group(s).
In addition, Silicic acid is an Inorganic acid (also known as mineral acid). Inorganic acids form hydrons and conjugate base ions when dissolved in water
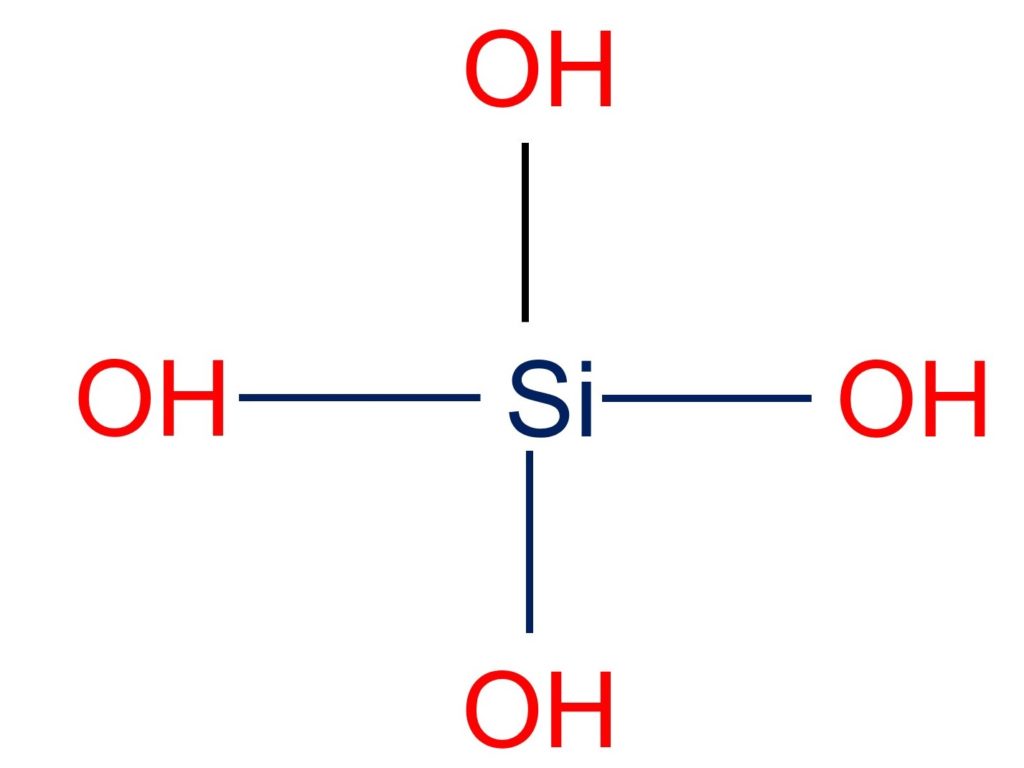
Silicic acids can be made by acidifying certain silicate salts in an aqueous solution, such as sodium silicate. They lose water when heated and create silica gel. Silicon exists largely in the seas as orthosilicic acid (H4SiO4), and its biogeochemical cycle is governed by a kind of algae known as “diatoms.”.
| Name of molecule | Silicic Acid ((H4SiO4)) |
| Physical form | powder/white |
| Molar mass | 96.113 g/mol |
| Density | 2 – 6 g/cm3 (20 °C) |
Table of Contents
Molar Mass of Silicic acid (H4SiO4)
The molar mass of Oxygen =15.9994 g/mol
Hydrogen molar mass = 1.00794 g/mol
Molar mass of Silicon= 28.0855 g/mol
Molar mass of H4SiO4 = 4(1.00794)+ 28.0855 +4(15.9994)
Molar mass of Silicic acid= 96.113 g/mol
Uses Of Silicic acid (H4SiO4)
- Several silicic acid esters are thermally stable liquids produced from alcohols and silicon tetrachloride that are used as lubricants and hydraulic fluids.
- Silicic acids can be made by acidifying certain silicate salts, such as sodium silicate, in an aqueous solution. When heated, they lose water and create silica gel. Silica gel is used to absorb moisture and condensation, which helps to keep things dry.
Silica Gel
Silica gel is a manufactured version of Silicon dioxide that functions as a desiccant in nature. Quartz and sand are both made of the same substance. It is capable of absorbing and retaining water vapor. Its packages are kept in any location that is prone to dampness.
Is Silicic Acid a strong acid?
Silicic acid is a very weak acid (pKa = 9.7–9.9)16,17 and the addition of acid to a solution of inorganic silicates results in the formation of Si(OH)4. Weak acids are acids that don’t completely dissociate in solution.
Silicon Dioxide (SiO2)
Silica (SiO2) is the most common and the most important compound of silicon.
In its molecular structure, every silicon atom is attached tetrahedrally to four oxygen (O) atoms.
Each O-atom has two close silicon neighbors.
It is the main constituent of more than 95 percent of the known rocks.
Earth’s crust is 59 percent silica.
Silica has three main crystalline varieties: quartz, tridymite, and cristobalite.
Related Links
Perchloric acid| Is It Super Acid?| 5 Key Points
HCN Lewis Structure| Step By Step Construction
SO2 (Sulfur Dioxide)
SiO2 Lewis Structure| Step By Step Construction
Hydrogen Bond| Definition and Easy Explanation
Frequently Asked Questions
1. Oxygen (O2) Polar or Nonpolar?
The oxygen (O2) molecule is nonpolar because it is diatomic and both atoms have similar electronegativity. Both oxygen atoms have equal charges, and there are no partial charges on either atom. As a result, O2 is a nonpolar molecule with a zero dipole moment.
2. Is carbon dioxide Polar or Nonpolar?
Carbon dioxide (CO2) is nonpolar due to its linear, symmetrical structure. The two oxygen atoms from either side of the carbon atom pull the electron density equally from both sides. CO2 is nonpolar in nature because there is no uneven sharing of valence electrons.
3. What is the magnesium element?
Magnesium (Mg) is a chemical element with an atomic weight of 24,312 and an atomic number of 12. It belongs to Periodic Table Group IIa. Magnesium is a metallic element with a silvery-white color. It has a relative density of 1740 kg/m3 (0.063 pounds per inch3 or 108.6 pounds per square foot). Because of its low weight and ability to make mechanically robust alloys, it has long been recognized as the lightest structural metal in the industry.
4. What is nitric acid?
Nitric acid (HNO3) is a colorless, fuming, and very corrosive liquid that is employed as a laboratory reagent as well as an important industrial chemical in the manufacture of fertilizers and explosives. It is toxic and can result in severe burns.
5. What is the process of Sublimation?
Sublimation is the process by which a solid turns straight into a gaseous state without first going through the liquid stage. Dry ice is one of several sublimation instances (frozen carbon dioxide). When dry ice is exposed to air, it immediately changes its phase from solid to gaseous (fog).
Vapor deposition is the inverse of sublimation.
6. Briefly define the process of Distillation?
Distillation is the separation of a mixture of liquids based on variations in their boiling points (or volatility). Water may be extracted from a salt solution using this method.
More Links
Is Carbon Dioxide a Pure Substance?
Is Chlorine a metal?
Electron Configuration for Calcium
Valence Electrons in Nitrogen
CH4 Lewis Structure & Molecular Geometry
Delocalized pi bond

Disclaimer
All information is for educational purposes and is the personal view of the authors; not intended as medical advice, diagnosis, or prescription. This information has not been evaluated by the FDA and is not intended to cure and prevent any disease.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



