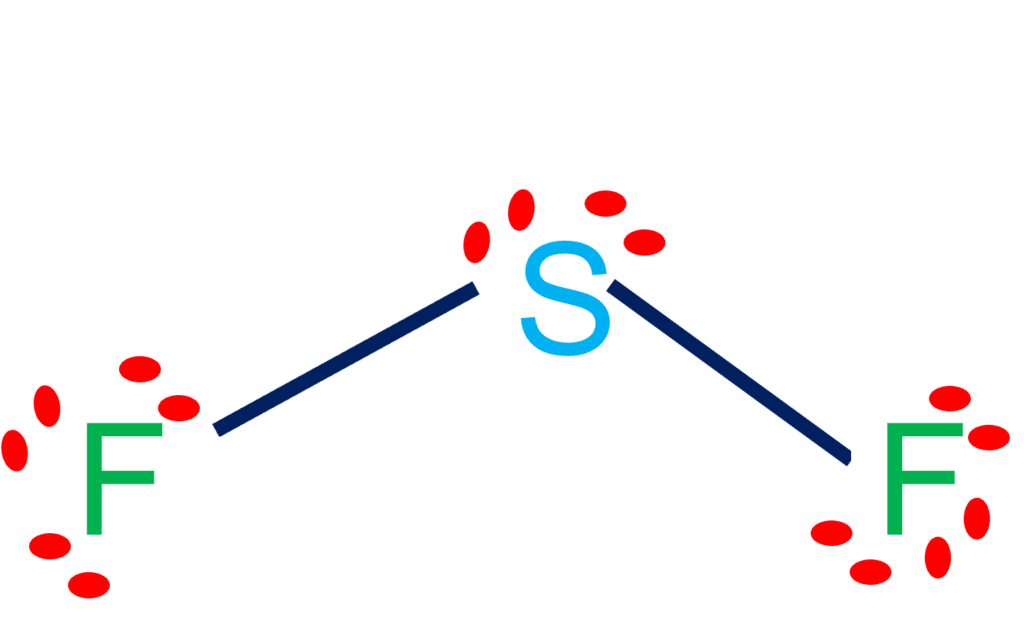
Sulfur difluoride (SF2) is a highly unstable inorganic compound. SF2 lewis structure comprises one Sulfur Atom and two Fluoride atoms. It is a polar molecule with bond angles of 98 degrees.
| Name of molecule | Sulfur difluoride (SF2) |
| Other names | sulfur difluoride, sulphur(II) fluoride sulfur difluoride, sulfur fluoride sulfur(II) fluoride, sulfur fluoride |
| Bond Angles | 98 degrees |
| Molecular Geometry of Sulfur difluoride | Bent |
| Hybridization of Sulfur difluoride | Sp3. hybridization |
| No Valence Electrons in the molecule | 20 valence electrons |
Table of Contents
Step by Step Construction of Lewis Structure
Following are the steps to construct the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw the Lewis structure, we need to figure out the sulfur electronic configuration and fluorine electronic configuration, and the number of valence electrons in individual atoms as shown in the table below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 16S | 1s2 2s2 2p6 3s2 3p4 | 6 |
| 9F | 1s22s22p5 | 7 |
VEs= VEs in sulfur + VEs in fluornie
Valence electrons = 6+2(7) = 20
Step-2: Determine the central atom
If we check the proper arrangement of Sulfur (S) and Fluorine (F) in the periodic table, we will find that the electronegativity values of S and F are 2.58 and 3.98 respectively.
As per the rule, the atom with the least electronegative value should be at the structure’s center. Since Sulfur is the least electronegative so it will be in the central position.
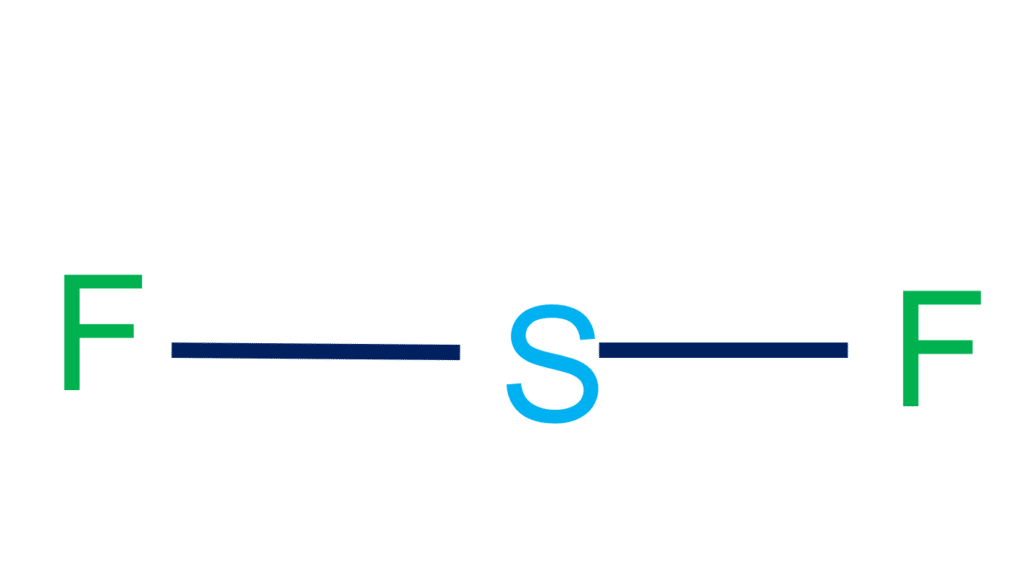
Step-3: Place electron pairs between the atoms
We need to distribute the 20 remaining valence electrons. Sulfur will have six electrons and fluorine will have seven electrons

Step-4: Place remaining electrons around the other atoms
After making a single bond with fluorine atoms sulfur is left with one lone pair of electrons.
SF2 Molecular Geometry
SF2 molecular geometry is bent in shape.
The molecule’s molecular geometry is determined by the Lewis structure and the placement of valence electrons in the structure. In SF2 lewis structure, the sulfur atom possesses two bonding pairs of electrons and two nonbonding pairs of electrons that reflect the VSEPR idea of AX2E2, which correlates to an angular/non-linear or bent molecular geometry. As a result, Sulfur Difluoride has a bent molecular geometry.
SF2 Lewis Structure- Key Points
- SF2 molecular geometry is bent.
- SF2 molecule is polar in nature.
- In the SF2 lewis structure, there is a single bond between sulfur and two fluorine atoms.
SF2 Hybridization
The electronic configuration of Sulfur is 1s2 2s2 2p6 3s2 3p4. First, the electrons are filled in 1s, then in 2s, and so on.
Similarly, the electronic configuration of Fluorine is 1s2 2s2 2p5. These configurations are decided on the basis of the number of electrons these elements have.
There are two sigma bonds, S-F and S-F.
To determine the Hybridization of this molecule, we take into account the number of atoms in the molecule as well as the total number of lone electron pairs bonded to the molecule. The Sulphur atom is bonded with two other atoms and contains two electron pairs in SF2 molecular geometry. The result is 4, which corresponds to sp3 Hybridization. Actually, both fluorine atoms are sp3 hybridized. As a result, SF2 possesses sp3 Hybridization.
Molar Mass of SF2
Molar mass of sulfur = 32.07 g g/mol
Molar mass of Fluorine= 19.00 g/mol
Molar mass of SF2 is 70.062 g/mol
Is SF2 Polar or Nonpolar?
SF2 is a polar molecule.
SF2 is polar in nature because the sulfur(2.58) and fluorine(3.98) atoms in the molecule have different electronegativity and the molecule has a bent geometrical form. As a result, the dipoles of the S-F bond do not cancel out, and molecules become polar and contribute some dipole moment.
Related Links
H2S Lewis Structure & Molecular Geometry
N2 Lewis Structure| Hybridization & Molecular Geometry
Is NH3 Acid or Base?| NH3 Properties
SO2 Lewis Structure| 4 Simple Steps
CO Lewis Structure & Molecular Geometry
Summary
To summarize everything in this article, the following are some important points:
- In the SF2 lewis structure, sulfur forms one single bond with each fluorine atom.
- The bond angle is 98 degrees and there are 20 valence electrons.
- SF2 is a polar molecule with bent geometry.
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below
1. Why is hydrogen cyanide polar?
In Hydrogen cyanide carbon has an electronegativity of 2.5, hydrogen’s electronegativity is 2.1, and nitrogen has an electronegativity of 3. Any molecule that has a difference in electronegativities of any dipole moment is considered polar. Therefore, Hydrogen cyanide is a polar molecule.
2. Explain Hydrogen Cyanide Lewis Structure in simple words
Hydrogen cyanide is a polar molecule with a triple bond between carbon and nitrogen. The structure is made up of three different atoms of hydrogen, carbon, and nitrogen. IT is a polar molecule with bond angles of 180 degrees.
3. What is hydrocyanic acid?
A solution of hydrogen cyanide in water is called hydrocyanic acid.
4. What is cyanide poisoning?
Cyanide poisoning refers to the harmful effects of inhaling hydrogen cyanide or ingesting the salts of hydrogen cyanide, called cyanides.
5. Why Lewis structures are important?
Lewis structure is a simplified representation of valence shell electrons.
It depicts the arrangement of electrons around individual atoms in a molecule.
Electrons are shown as “dots” or as a line between two atoms when they are bonded.
6. How to draw Lewis’s structure of oxygen?
In the O2 Lewis structure, there is a double bond between two oxygen atoms.
Oxygen is a diatomic nonpolar molecule with bond angles of 180 degrees.
In its molecule, both oxygen atoms have the same electronegativity value and both atoms share equal ratios of bonded shared electrons and the overall O2 molecule turns out to be nonpolar.
7. What is the dot structure of Hydrogen Sulfide?
On both sides of the central sulfur atom in the H2S Lewis structure, there are two hydrogen atoms.
The molecule bends due to the existence of two unbonded pairs of electrons.
The molecule is slightly polar because sulfur is more electronegative than hydrogen.
In the case of H2S, the vectorial sum of the bond dipole moments results in a non-zero total dipole moment. As a result, dipole-dipole interactions are observed in hydrogen sulfide.
8. What is CLF3 molecular geometry?
ClF3 has a T-shaped molecular geometry and trigonal bipyramidal electron geometry. This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. ClF3 is a polar compound.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
More Links
SF4 Lewis Structure & Molecular Geometry
HCN Lewis Structure & Molecular Geometry
O3 Lewis Structure | Step By Step Drawing
N2O Lewis Structure| Laughing Gas
Co2 Polar or Nonpolar
Is HCl Polar or Nonpolar?
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023