Atmospheric pressure is the pressure exerted on objects from the air or the Earth’s atmosphere.
The average value for the atmospheric pressure at sea level is defined as 1 atmosphere (atm).

Atmospheric Pressure in Simple Words
Our earth is surrounded by a cover of air which is called the atmosphere. It ends at a few hundred kilometres above sea level.
Just as certain sea creatures live at the bottom of an ocean. We live at the bottom of a vast ocean of air. This ocean of air exerts pressure on objects present at the surface of the earth, this pressure is called atmospheric pressure.
Air is a mixture of gases with varying densities. Its density decreases continuously as we go up.
The average value for the atmospheric pressure at sea level is defined as 1 atmosphere (atm).


Atmospheric Pressure Definition
Atmospheric pressure is defined as the force per unit area at a given location on the Earth caused by the weight of the air above it. At sea level, this pressure is 101.3 kPa in SI units.
Examples of Atmospheric Pressure
The bubbles of soap are spherical because the air pressure inside the bubble is equal to the atmospheric pressure. Similarly, the balloon expands as we fill air into it. The air pressure inside the balloon is equal to the atmospheric pressure.

What is Pressure?
The force acting normally per unit area on the surface of a body is called pressure. In other words, the pressure is force distributed over an area. Its unit is the Pascal.
What is Pascal?
The pressure is one Pascal if one Newton force is applied perpendicularly upon an area of a 1-meter square. In SI units, one Pascal is one kilogram per meter per second squared.
Units
- Pascal (MKS system).
- N/m2 =1 Pa.
- bar = 105 Pa.
- atm =101325 Pa.
- Torr = 133.322 Pa.
- Foot-pound force/inch2 = 6894.76 Pa.
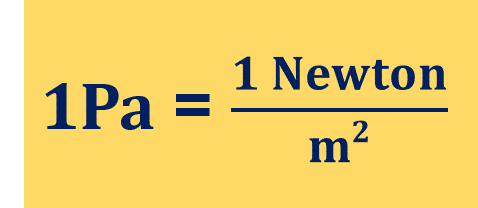
Barometric Pressure
Barometric pressure, also known as atmospheric pressure, is the force exerted by the weight of the Earth’s atmosphere on objects on its surface. It is the pressure measured by a barometer and is expressed in units such as millibars (mb) or inches of mercury (inHg).
Barometric pressure can be affected by various factors such as altitude, temperature, humidity, and weather conditions. In general, as altitude increases, the pressure decreases. Changes in temperature, humidity, and weather patterns can also affect the pressure, resulting in changes in the weather.
Barometric pressure is an important parameter in weather forecasting as it provides information about current and future weather conditions. For example, a rapid decrease in barometric pressure often indicates an approaching storm or low-pressure system, while a rapid increase in pressure can indicate improving weather conditions.
Bar to Pascal Conversion
The bar to pascal [Pa] conversion relation is as follows:
1 bar = 100000 Pascals = 105 Pascals = 100 kPa.
Key Points
- The perpendicular force acts normally per unit area on the surface of a body.
- Pressure is Pascal (N/m2).
- Pressure depends on Force (F) acting normally and the Surface area (A) on which force is applied.
- The pressure is a scalar quantity.
- One pascal is a fairly small amount of pressure. It takes 101,325 pascals to equal one atmosphere.
- Bar to Pascal conversion: 1 bar is 105 pascals.

Real life Examples of Pressure
Some daily life examples of pressure are:
- Air pressure (psi) in a car tire supports the weight of the car.
- Fluid flows in a straw due to the difference in air pressure.
- In Aircraft, the air pressure difference between the top and bottom of the wing creates a force that lifts the wing up into the air.
- A bullet fired from a gun is driven by the sudden high pressure of generated gases.
- The balloon inflates because of the air pressure inside it.

What is Newton?
One Newton (N) is equal to the force that would give a mass of one kilogram an acceleration of one meter per second per second.
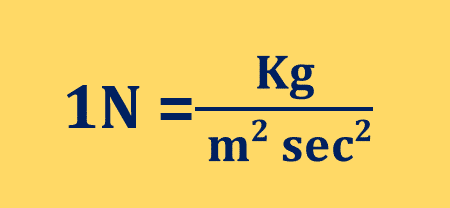
Daily Life Examples of Atmospheric Pressure
Here are some examples of atmospheric pressure in daily life:
- Drinking through a straw: When you suck on a straw, you create a lower pressure area inside the straw. The higher atmospheric pressure outside the straw pushes the liquid up and into your mouth.
- Weather changes: Atmospheric pressure affects the weather. Low-pressure systems often bring rainy and stormy weather, while high-pressure systems tend to bring clear skies and calm weather.
- Inflating a tire: When you inflate a tire, you increase the air pressure inside the tire. The atmospheric pressure outside the tire pushes back against the tire, keeping it inflated.
- Altitude sickness: As you go to higher altitudes, the atmospheric pressure decreases. This can cause altitude sickness as your body adjusts to the lower pressure.
- Boiling water: When you boil water, the heat causes the water molecules to move faster and turn into steam. The steam has a higher pressure than the surrounding air, which is why it rises and escapes the pot.
- Ear popping: As an aero plane ascends or descends, the atmospheric pressure changes rapidly. This can cause your ears to feel uncomfortable or pop as your body tries to adjust to the pressure changes.
- Vacuum cleaner: A vacuum cleaner works by creating a low-pressure area inside the vacuum tube, which sucks up dust and debris from the surrounding higher-pressure air.
What is Liquid Pressure?
Liquid Pressure (PL) is the pressure of a liquid on the surface of its container. It increases with the increase in the depth of liquid.
This pressure is more at the bottom as the liquid at lower depths has to support all of the water above it. Liquid pressure is a product of the density of liquid, gravity, and depth of liquid.

Properties of Liquid Pressure
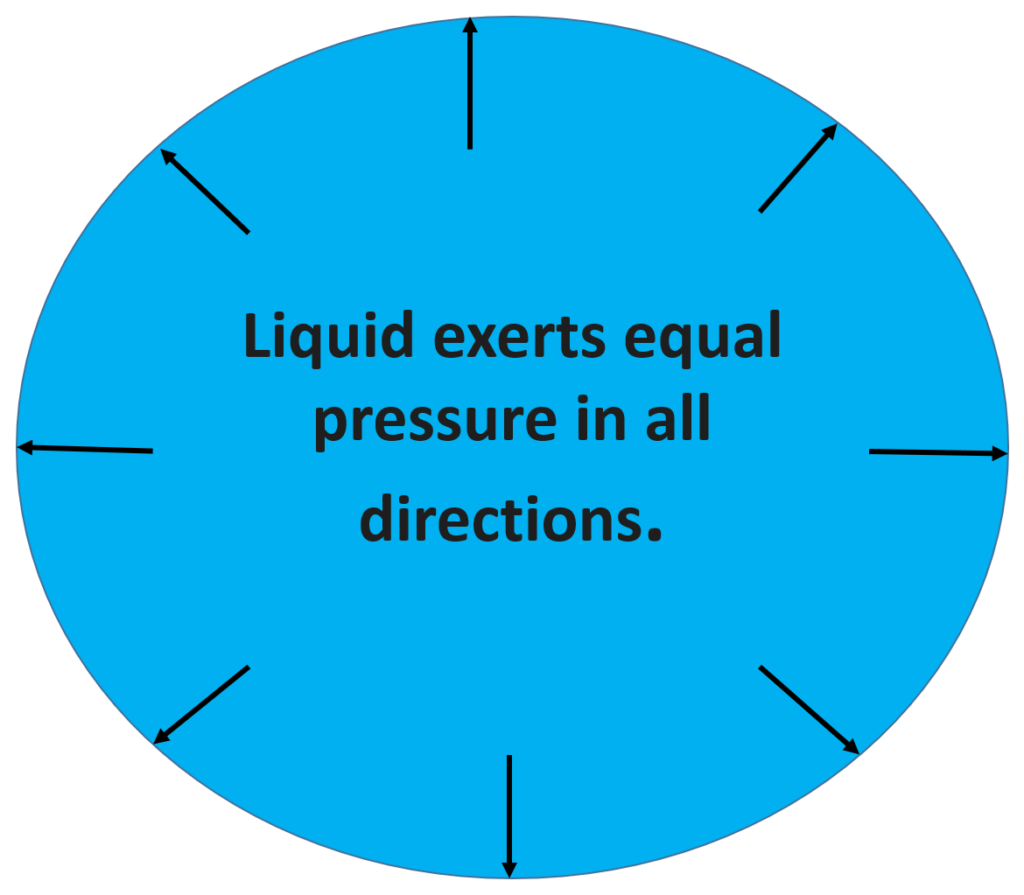
- Liquid exerts equal pressure in all directions.
- Liquid pressure depends upon the gravity, density, and depth of the liquid.
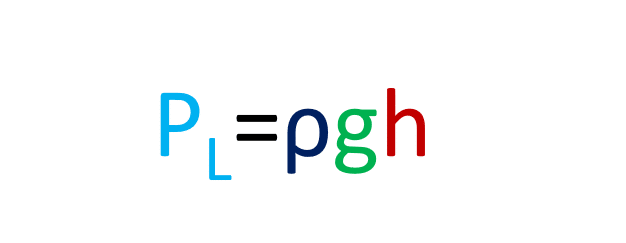
- Mathematically PL =ρgh, where ρ is the density of the liquid, g is gravity, and h is the height of the liquid column.
- Liquid pressure varies with the depth of the liquid.
Pascal’s law
Pascal’s law states that “If the pressure is exerted on a liquid, it is transmitted equally in all parts of liquid in all directions”.
Pascal’s law finds numerous applications in our daily life such as automobiles, hydraulic brake systems, hydraulic jack systems, hydraulic presses, and other hydraulic machines.
Example of Pascal Law: Consider a glass vessel having holes all over its surface. Fill it with water and push the piston. The force on the piston exerts pressure on the water. This pressure is transmitted equally throughout the liquid in all directions.
The water rushes out of the holes in the vessel with some pressure.
Pascal’s law is applicable for fluids for liquids as well as gases.
Derivation of Equation of Liquid Pressure
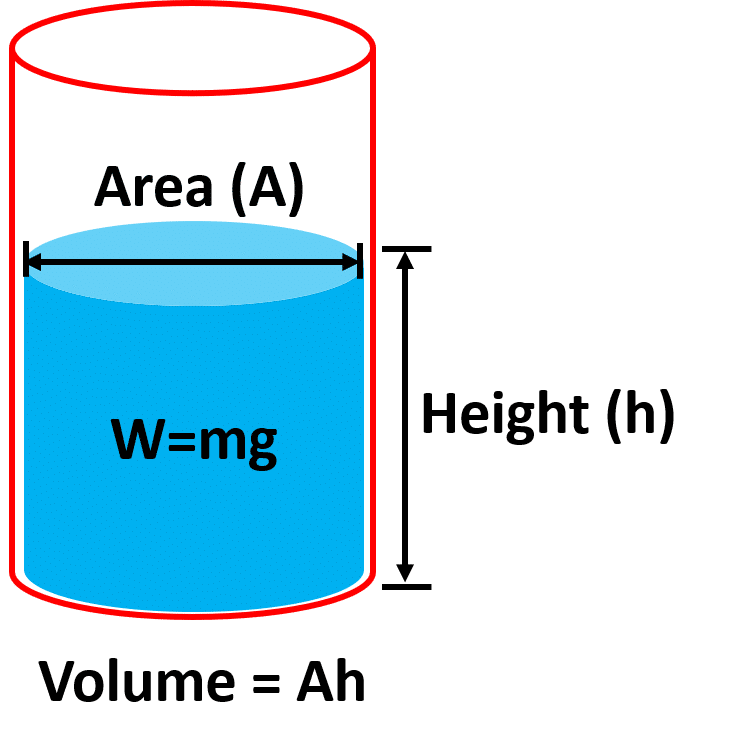
Consider a surface of area A in a liquid at depth h.
The length of the cylinder of liquid over this surface will be h.
The force (F) acting on this surface will be the weight w of the liquid above this surface.
if ρ is the density of the liquid and m is the mass of the liquid.
Mass of liquid in cylinder = volume x density.
m=(Axh)xρ
Force =ma (Newton’s law of motion)
F=mg (assuming acceleration equal to gravity)
F=Ahρg ……………………………..(1)
P=F/A (Definition of Pressure)
F=PA …………………………………..(2)
Comparing equation 1 and equation 2.
Ahρg=PA
P=ρgh
This equation shows that pressure in the liquid increases with the density of the liquid and depth of the water column.
Quick Links
- What causes atmospheric pressure?
- List of Pressure Measuring Devices.
- Definition of work
- Momentum relation
Examples
Example 1: A student presses her palm by her thumb with a force of 75 N. How much would be the pressure under her thumb to have a contact area of 1.5 x 10-4?
P=F/A
P= 75 N/(1.5×10-4)
P = 5 x 105 Nm-2
Example 2: If an object weighs 50 N and is lying on a side with an area of 2m by 2m, what is the pressure exerted on the surface?
Pressure = Force ÷ Area.
P = 50 N ÷ (2m x 2m).
P = 50 N ÷ (4m2).
Pressure= 12 pascals
Related Links
Can Kinetic Energy Be Negative?
Instantaneous Velocity
Room Temperature
Light Energy
Mechanical Energy
Gauge Pressure Formula
Frequently Asked Questions (FAQs)
1. What is a state function?
A state function is a property that depends on the state of a system and is independent of the path taken to get it. Pressure and temperature, for example, are state functions.
2. What is air?
Air is a homogeneous mixture of different gases. The air in the atmosphere is composed of nitrogen, oxygen (which is required for animal and human life), carbon dioxide, water vapor, and trace amounts of other elements (argon, neon, etc.). At higher elevations, the air contains ozone, helium, and hydrogen.
3. Linear motion?
Linear motion (also known as rectilinear motion) is a one-dimensional motion along a straight line that can be represented mathematically with only one spatial dimension. Linear motion is defined as movement along a straight path, whereas nonlinear motion is any movement that is not in a straight line.
4. instantaneous velocity?
Instantaneous velocity is defined as the rate of change of velocity during a very short period of time.
5. Linear acceleration?
Linear acceleration occurs when the velocity of an object travelling in a straight line increases or decreases over time.
6. What is a pressure gradient?
In fluid dynamics, the pressure gradient is used to describe the force that drives the fluid flow. The gradient is usually measured in units of force per unit distance, such as newtons per meter (N/m), and it is used to calculate the velocity of fluid flow.
1MPa = 1000000 Pa = 1 x 106 Pa = 1 million Pa.
1 MPa = 145.038 psi.
1 MPa = 1000 kPa.
1MPa = 1000000 Pa
1 Pa =1/1000000 MPa
1 Pa = 10-6 MPa.
When an object is immersed in a liquid, then there are two forces act on the object.
Its weight acts in a downward direction.
An upthrust that acts in the upward direction.
1MPa = 1000000 Pa
100 MPa = 108 Pa.
1MPa = 1000000 Pa
1 Pa = 10-6 MPa.
101325 Pa =10.1325 MPa
The fan in a vacuum cleaner lowers the air pressure in its bucket. The atmospheric air rushes into it carrying dust and dirt with it through its intake port. The dust and dirt particles are blocked by the filter while the air escapes out.
When air is sucked through a straw, the air pressure within the straw decreases. This causes the atmospheric pressure to push the liquid up the straw.
The pressure of air in a car tire is 32 pis.
1 Pa = 0.000145038 psi (pounds/ square inch).
32 psi =220.632 kPa
Pressure on our body at sea level = 14.7 pounds per square inch.
Pressure increases as you go down under the ocean.
More Links
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
- H2S Polar Or Nonpolar| Easy Explanation - April 5, 2021
- Atmospheric Pressure| Definition & Real-Life Examples - January 9, 2021
- Molar Mass of Acetic Acid| Easy-Explanation - January 8, 2021