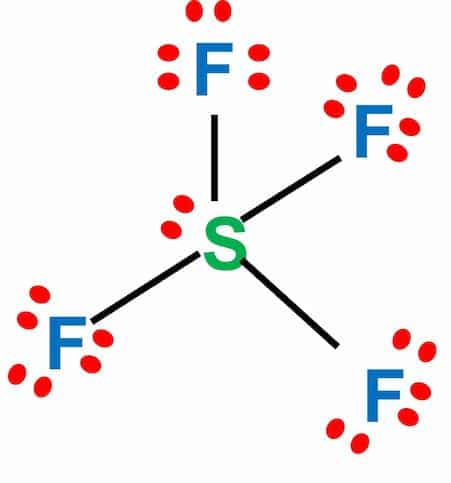
Sulfur Tetrafluoride (SF4) is a colourless corrosive gas used to synthesize several organofluorine compounds. SF4 lewis structure comprises one sulfur and four fluorine atoms. The central sulfur atom is linked to four fluorine atoms and contains one lone pair.
SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses.
It has trigonal bipyramidal molecular geometry.
| Name of molecule | Sulfur Tetrafluoride (SF4) |
| Bond Angles | 102o and 173o |
| SF4 Molecular geometry | trigonal bipyramidal |
| The polarity of the SF4 molecule | Polar |
| No of Valence Electrons in SF4 molecule | 34 valence electrons |
Table of Contents
SF4 Lewis Structure-Step by Step Construction
Following are the steps to construct the Lewis Structure.
Step-1: Count the valence electrons of atoms
To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below.
| Atom | Electronic Configuration | Valence Electrons (VEs) |
| 16S | 1s22s22p63s23p4 | 6 |
| 9F | 1s22s22p5 | 7 |
Valence electrons in SF4 = 6+ 4(7) = 34 valence electrons
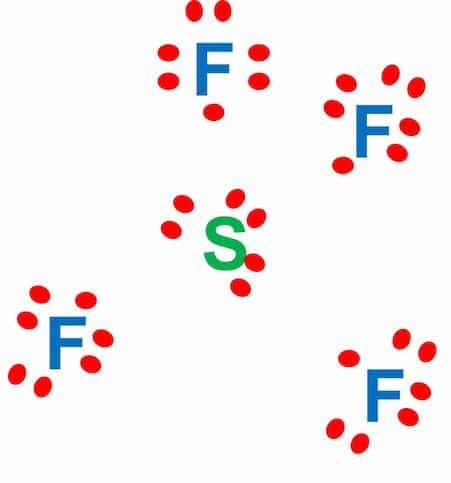
Step-2: Place electron pairs between the atoms
The Lewis diagram of SF4 shows four fluorine atoms having seven dots of valence electrons. Where six electrons are arranged around each sulfur atom
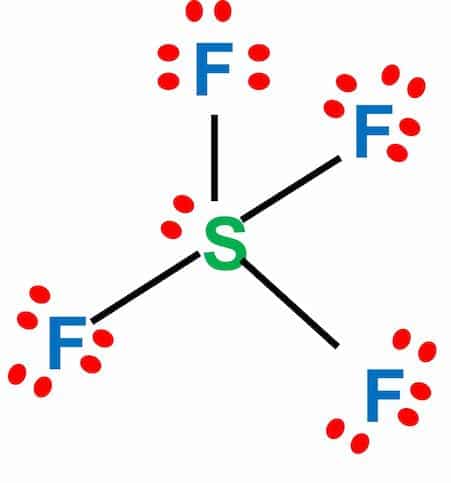
Step-3: Place remaining electrons around the other atoms
The sulfur atom will form bonds with each of the fluorine atoms, requiring eight valence electrons. The octet of four fluorine atoms will have three lone pairs of electrons and will use 24 valence electrons. In addition, two electrons will be kept as lone pairs in the sulfur atom. The resulting bond is a covalent bond.
SF4 Molecular Geometry
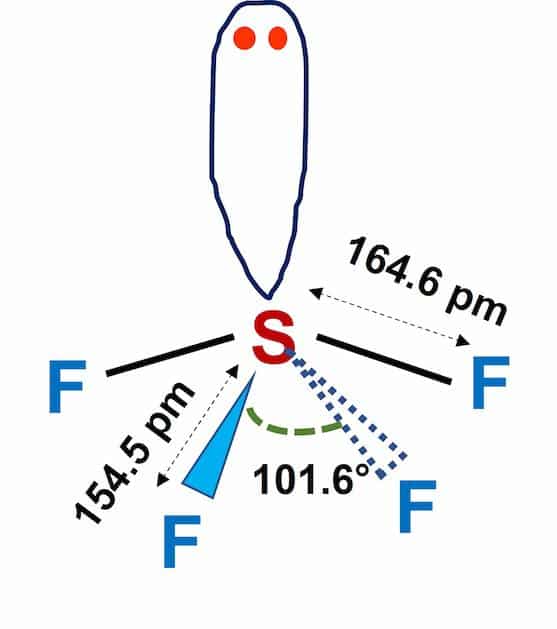
According to its chemical formula and hybridization, the molecular geometry of SF4 is trigonal bipyramidal. The shape resembles a see-saw in general, and this shape is caused by repulsion in bonding and lone pairs of electrons.
Instead of the normal 120° bond angle, the equatorial fluorine atoms have 102° bond angles. Instead of the real 180° bond angle, the axial fluorine atom angle is 173°.
SF4 Lewis Structure- Key Points
- In the SF4 Lewis structure, the central sulfur atom has one lone pair and is bonded to four fluorine atoms.
- SF4 molecule consists of a total of 34 valence electrons
- Density = 1.95 g/cm³
- The boiling point is -38 °C
- Melting point −121.0 °C
- The molar mass of SF4 is 108.07 g/mol.
- SF4 molecule is polar in nature

Uses of Sulfur Tetrafluoride
- Sulfur Tetrafluoride is a colorless gas. It is utilized as a fluorinating agent as well as in the production of water and oil repellent polymers. It is also utilized in the production of insecticides. Because it has been cited by ACGIH, DOT, NIOSH, DEP, and EPA, sulfur tetrafluoride is on the Hazardous Substance List.
Is SF4 Polar or Nonpolar?
SF4 is polar in nature because the sulfur atom has a lone pair on it, causing the molecule’s structure to become asymmetric, i.e. seesaw.
SF4 Hybridization
- The SF4 molecule consists of 34 valence electrons. Six electrons will come from sulfur, and each of the four fluorine atoms will have seven.
- The electronic configuration of the sulfur atom (Z=16) is 1s2 , 2s2 , 2p6 , 3s2 , 3p4
- The electronic configuration of fluorine is 1s2, 2s2, 2p5
- The bonding between sulfur and fluorine results in the creation of four single bonds and one lone pair in sulfur. Based on this, we may conclude that the number of electron density areas is 5.
- The five valence atomic orbitals in the center S atom are basically hybridized to create five sp3d hybrid orbitals.
- Four hybrid orbitals overlap in 2P-orbitals, with the fifth containing a lone pair.
- Sulfur will have five orbitals, one of which is a 3s orbital, three 3p orbitals, and one 3d orbital.
Summary
To summarize everything in this article, the following are some important points:
- In the SF4 Lewis structure, the central sulfur atom has one lone pair and is bonded to four fluorine atoms.
- The bond angle is 180 degrees and there are 12 valence electrons.
- SF4 is a polar molecule with bipyramidal geometry.
- There are thirteen lone pairs of electrons in the sulfur tetrafluoride molecule.
More Links
CO2 Lewis Structure and Molecular Geometry
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
CO Lewis Structure & Molecular Geometry
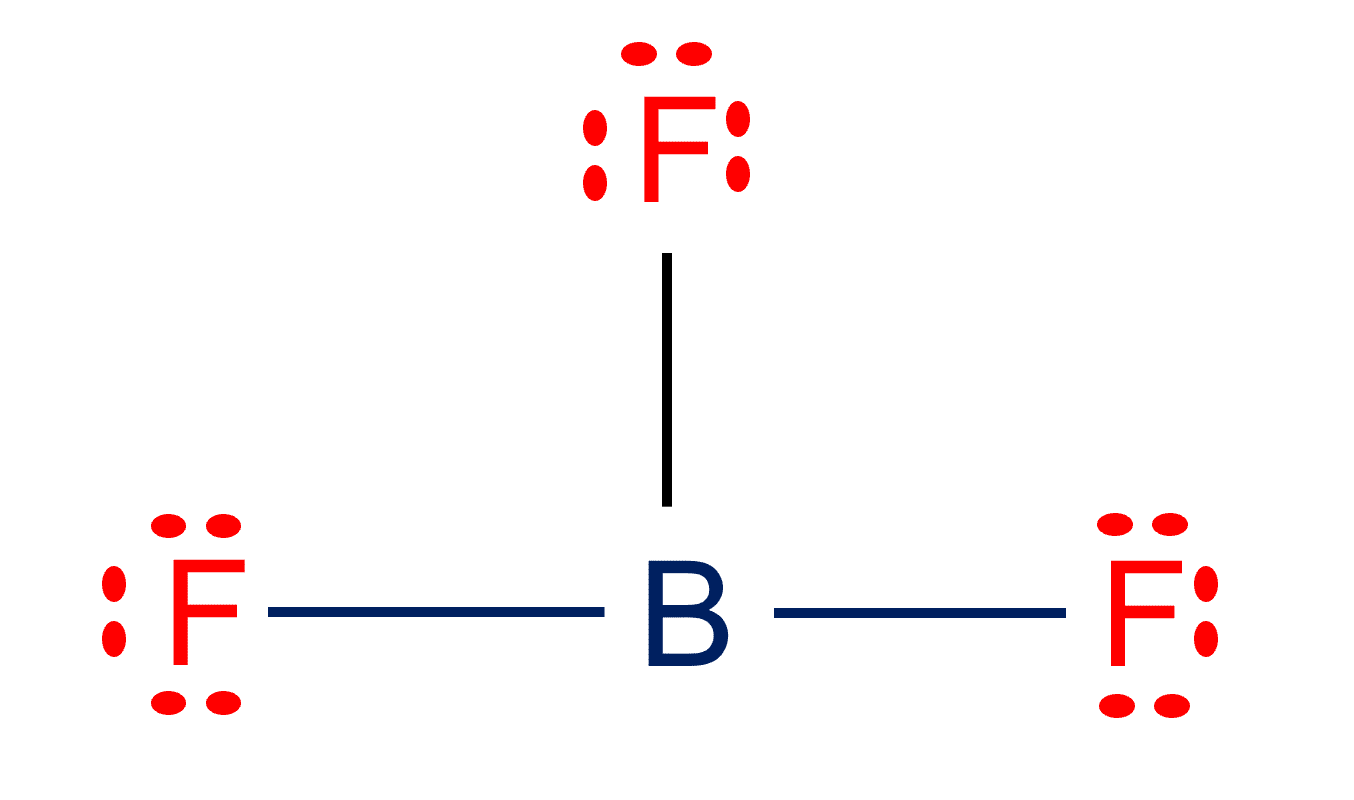
BF3 Lewis structure| Molecular geometry, Hybridization
HCN Lewis Structure & Molecular Geometry
Frequently Asked Questions (FAQs)
1. How to draw the lewis structure of SO2?
SO2 Lewis structure would comprise two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six. The total number of SO2 valence electrons is 12.
- The molecular geometry of SO2 is a trigonal planner.
- The three pairs of bonding electrons are arranged in the plane at an angle of 120-degree.
- The sulfur’s valence electron = 6
- valence electrons of oxygen in SO2 are 6
2. What are unbonded pairs of electrons?
Unbonded pairs of electrons are unshared valence electrons.
They are also called lone pairs of electrons.
They are found in the outermost electron shell of atoms and can be identified by drawing Lewis structure.
3. What is SO2?
SO2 (Sulfur dioxide) is the entity of a bond between Sulfur and Oxygen atoms.
It is a colorless, toxic, and inorganic gas with a pungent smell like Nitric acid.
SO2 gives a weak acid solution when dissolved in water.
It is naturally found in small amounts in the atmosphere and is a primary precursor of Sulfuric acid.
4. What are the uses of oxygen?
Production of steel, plastics, and textiles, brazing, welding, and cutting of steel and other metals, rocket propellant, oxygen therapy, and life support systems in airplanes, submarines, spaceflight, and diving are all examples of common applications of oxygen.
Because of its linear, symmetrical form, carbon dioxide (CO2) is nonpolar. The electron density is drawn equally from both sides by the two oxygen atoms in either direction of the carbon atom. CO2 is nonpolar in nature because there is no uneven sharing of valence electrons.
The gas sulfur dioxide (SO2) has a polar character. It’s a polar molecule because of the electronegativity mismatch between the sulfur (2.58) and oxygen (3.44) atoms. Additionally, SO2 has a bent shape due to the existence of unbonded electrons on the sulfur and oxygen atoms.
More Interesting Links
Hydrogen Bond| Definition & Easy Explanation
Is Titanium Magnetic?
Bromine Trifluoride(BrF3)|Bond Angle & Hybridization
Sulfurous Acid Formula & Lewis Structure
N2 Lewis Structure| Hybridization & Molecular Geometry
Is BF3 Polar or Nonpolar?
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023