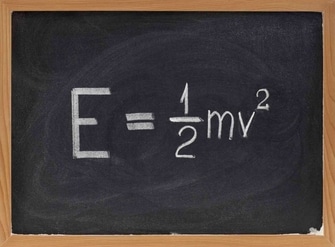Light is described as electromagnetic radiation released by hot things such as lasers, bulbs, and the sun. Light energy is a sort of kinetic energy that has the power to make different types of light visible to the human eye.
Light can also be described as a flow of particle-like ‘wave-packets,’ known as photons, that travel at the speed of 300,000 kilometers per second. The term energy of light refers to the energy of these photons, which is calculated by this equation, E = hf, where E is the energy of the photon in Joules; h is Planck’s constant, which is always 6.63 * 10-34 Joule seconds; and f is the frequency of the light in hertz.
The Sun is the closest star to the planet Earth and radiates light energy.
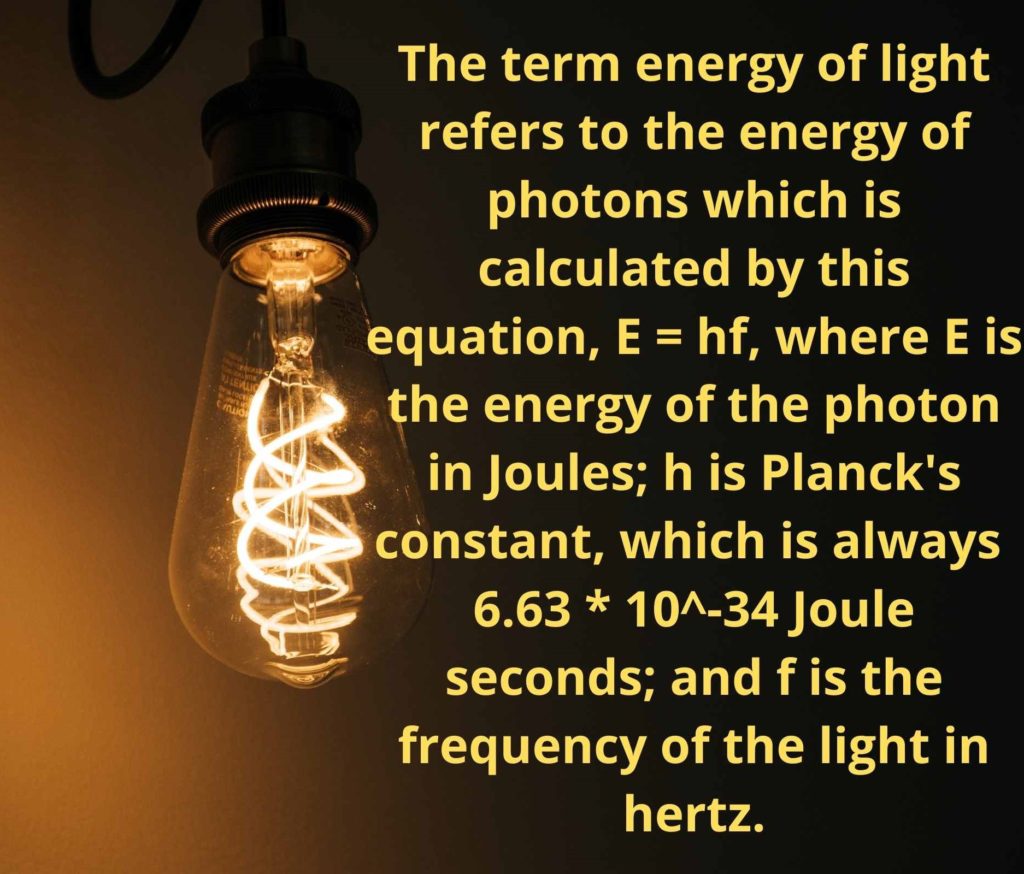
Table of Contents
What is Light?
Light is technically any form of radiant energy but is almost understood as visible radiant energy. This visible radiant energy has wavelengths ranging from 380 nm (one billionth of a meter) to 750 nm. Figure 1 shows the visible spectrum of visible light. Light is a type of electromagnetic radiation, which means it has electric and magnetic fields that vibrate back and forth like a wave. Because of the peculiarities of quantum mechanics, light is also considered as packets of tiny particles with no mass, known as photons. Photons are fundamental quantum objects like electrons and neutrons. A single photon, the smallest unit of light, carries properties such as wavelength, frequency, wavenumber, wavevector, period, speed, wave phase, momentum, spin, and quantized electromagnetic field.

Light Energy Formula (E=hf)
A photon’s energy (light energy) E equals its frequency f multiplied by a constant, E = hf.
Where:
- E = Energy of the photon (in Joules).
- h = Planck’s constant.
- f = frequency of the light in units of per second (1/seconds)
Another version of the light energy formula or photon energy formula is related to the wavelength of light which is given as, E = hc/λ.
Where:
- E = Energy of the photon (in Joules)
- h = Planck’s constant.
- c = speed of light, another constant.
- λ = wavelength of the light, usually in units of meters
Some important terms related to the energy of light are the photon’s momentum, time period, frequency, wavelength, and speed of light.
A photon’s momentum p equals its wavevector k multiplied by a constant, p = ℏk. Furthermore, the period T is simply the inverse of the linear frequency f, T = 1/f, the wavelength is simply the inverse of the wavevector magnitude k times 2π, λ = 2π/k, and the speed c is simply the frequency times the wavelength, c = fλ.
Uses of Light Energy
Among millions of uses of light energy, a few are listed below:
- Light helps us see things (Photonics).
- Plants and algae, for example, use light energy to make food energy by combining carbon dioxide and water (Photosynthesis).
- Satellites use light to power themselves.
- A solar thermal power plant captures and focuses sunlight on producing the high-temperature heat required for electricity generation.
- Solar power technologies use photovoltaic panels or mirrors to focus solar radiation to convert sunlight into electrical energy.
Summary
- The energy of light is equal to the energy of photons. The light energy formula is given as E = hf, where E is the photon’s energy in Joules, h is Planck’s constant, which is always 6.63 * 10-34 Joule seconds, and f is the frequency of light in hertz.
- Visible light has wavelengths ranging from 380 nm to 750 nm.
Related Topics
Greenhouse Effect
Energy Stored in a Capacitor
Relativistic Kinetic Energy
Thermal Energy Equation
Kinetic Energy Formula
Frequently Asked Questions (FAQs)
1. What is white light?
White light is a nearly equal mixture of all colors (red, orange, yellow, green, blue, and violet). For humans, white things appear white because they reflect all visible wavelengths of light that shines on them, giving the light a white appearance. Colored objects, on the other hand, only reflect a portion of the wavelengths; the remainder is absorbed.
2. What are electromagnetic waves?
Electromagnetic waves are produced as a result of a periodic change in an electric or magnetic field. Radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, and X-rays, are examples of electromagnetic waves.
3. What is the ultimate source of energy?
The Sun is both the ultimate source of energy and our primary source of energy. For plants, the primary productivity (photosynthesis) is driven by sunlight, and all of our fossil fuels are derived from plants that lived millions of years ago.
4. What is the body’s preferred source of energy?
Carbohydrates are the body’s preferred source of energy. Carbohydrates, along with protein and fat, are one of three macronutrients. When you eat carbohydrates, they enter your bloodstream in the form of glucose, which is the body’s preferred energy source.
5. What is the crest of a wave?
The crest of a wave is the portion of the transverse wave above the mean level. The trough of a transverse wave is the section of the wave below the mean level. The wavelength is defined as the distance between two consecutive crests or troughs. Continue reading to learn more about the crest of a wave, amplitude, frequency, and wave types.
6. What are examples of light energy?
Light energy examples are sunlight, laser light, light from a glowing bulb, kerosene lamplight, light from stars, and other luminous bodies.
7. What is microfarad?
A word microfarad is a capacitance unit that is 0.000001 farad, or one-millionth of a farad. A microfarad is represented by the sign µF. The term is frequently utilized in alternating-current and audio frequency circuit applications as the capacitors found in them normally have a rating of 1 µF or more.
8. strain energy?
Strain energy is a type of potential energy stored in an item as a result of elastic deformation. When an item is deformed from its unstressed state, the external work done on it is turned into (and is considered equivalent to) the strain energy contained in it. It is measured in N-m or Joules.
9. What are power units?
Power is defined as energy divided by time. The unit of power in the International System of Units (SI) is the watt (W), which is equal to one joule per second.
10. What is surface charge density?
Surface charge density (σ) is the amount of charge per unit area, measured in coulombs per square meter (C⋅m−2), at any point in a two-dimensional surface charge distribution.
More Interesting Topics
Mechanical Energy Formula & Examples
What is a State Function?
Unit Weight of Water
Velocity Formula & Definition
Centripetal Acceleration| 9- Easy Examples
Longitudinal Waves| Physics
Reference
- P. Ronan, Gringer. (2013, Feb. 19). EM spectrum revised [Online]. Available: http://upload.wikimedia.org/wikipedia/commons/3/30/EM_spectrumrevised.png
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023