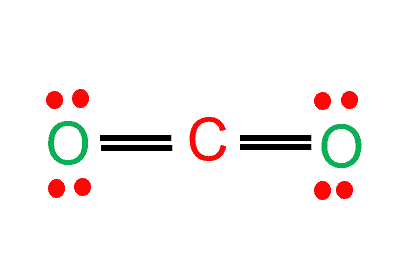
Pi bond (π bond) is defined as a bond formed by the overlap of p orbitals on adjacent atoms, perpendicular to any sigma bond(s) between the same atoms. For instance, Acetylene is said to have three sigma bonds and two pi bonds.
A pi bond is a covalent bond that is formed by the lateral overlapping of the half-filled atomic orbitals of atoms.
The pi bond is the “second” bond of the double bonds formed by carbon atoms, and it is represented as an elongated green lobe that extends above and below the plane of the molecule.
Pi bonds are formed when two parallelly oriented pi orbitals of adjacent atoms overlap sideways. Overlap in pi bonds occurs at the side of the two lobes of p–orbitals, so the extent of overlapping is less than in sigma bonds. Pi bonds are thus weaker than sigma bonds.
The pi bond is divided into two halves, one above and one below the plane of the molecule.
Table of Contents
Pi Bonds-Key Points
- Double and triple bonds between atoms are often composed of a single sigma bond and one or two pi bonds.
- The pi bond is a covalent bond created by the lateral overlap of atomic orbitals. For example, the ethylene molecule has 5 sigma bonds and 1 pi bond.
- The pi bond is weak due to the lower degree of overlapping. It is only possible between two ‘p’ orbitals with lower bond energy.
- The pi bond always forms when the sigma bond already exists.
Strength of Pi Bonds
Almost often, pi bonds are weaker than sigma ones. A carbon-carbon single bond (sigma bond), for example, has double the bond energy of a carbon-carbon double bond (sigma and pi bond).
The relative weakness of these bonds in comparison to sigma bonds may be explained by the quantum mechanical perspective that there is a substantially smaller degree of overlapping of p orbitals in pi bonds due to their parallel orientation. Sigma bonds, on the other hand, have a far higher degree of overlapping and are so likely to be stronger than the equivalent bonds.
More Links
Magnetic Permeability
Ampere-Electric Current Unit
Paramagnetism| Definition, and Examples
Power Units- The Basics
Surface Charge density
London Dispersion Force| Definition and Examples
Charge of Ammonia (NH3)| Simple Steps
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023