London dispersion force is the weakest type of intermolecular force caused by the uneven distribution of electrons in molecules. On average, electrons are evenly distributed in a molecule, but they can be clustered on one side of a molecule. This clustering results in an instantaneous dipole. The instantaneous dipole can cause a temporary dipole to form on surrounding molecules. The dipoles on each molecule then exert a force on each other.
In addition, intermolecular forces are the forces of attraction or repulsion that exist between neighbouring atoms, molecules, or ions. When compared to intramolecular forces, such as covalent or ionic bonds between atoms in a molecule, these forces are weak.
Intermolecular forces are classified into three types:
- London dispersion forces
- Dipole-dipole interactions
- Hydrogen bonding.

Table of Contents
London dispersion force Definition
London dispersion force refers to the weak intermolecular forces between atoms or nonpolar molecules due to temporary dipoles created by electrons’ motion. These forces are observed in nonpolar molecules such as halogens: fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2).
London dispersion forces are also known as dispersion forces, instantaneous dipole forces, or induced dipole forces.
London dispersion forces are caused by the fluctuating dipoles in a molecule, where the distribution of electrons is uneven and temporarily creates a positive and negative charge in different parts of the molecule. When two such molecules come close to each other, the fluctuating dipoles interact, creating an attractive force between the molecules.
London Dispersion force in Simple Terms with Examples
London dispersion forces are the weakest type of intermolecular forces and are also known as “induced dipole-induced dipole” forces. They arise due to the temporary alignment of electron clouds in neighbouring molecules, which results in the creation of small temporary dipoles.
Here are a few examples of London dispersion forces in action:
- The attraction between a nonpolar molecule such as nitrogen (N2) and another nitrogen molecule.
- The attraction between a nonpolar molecule such as hexane (C6H14) and another hexane molecule.
- The attraction between a polar molecule such as water (H2O) and a nonpolar molecule such as hexane.
In general, the strength of London dispersion forces increases as the size of a molecule increases due to the increase in electron cloud size and the greater opportunity for temporary dipoles to form. This is why nonpolar gases, like neon and xenon, tend to condense into liquids at relatively high temperatures compared to other gases of similar molecular weight.
Dipole-Dipole Interations
Dipole-dipole forces are attractive forces that exist between the positive and negative ends of polar molecules. They are much weaker than ionic or covalent bonds and only have an effect when the molecules involved are close together.
For instance, When two HCl molecules are brought close together, the positive H of one attracts the negative Cl of the other and forms a bond. Water (H2O): two hydrogen (H) atoms are bonded to an oxygen (O) atom in H2O. As a result, the O-H bond becomes permanently dipolar.
Furthermore, the primary distinction between dipole-dipole and London dispersion forces is that dipole-dipole forces occur among molecules with dipole moments, whereas London dispersions occur due to atoms or nonpolar molecules forming instantaneous dipoles.
Hydrogen Bonds
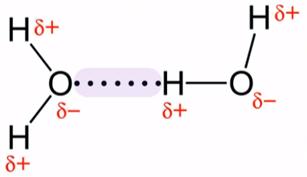
A hydrogen bond (H-bond) is a type of intermolecular force which develops when a hydrogen (H) atom bonded to a strongly electronegative atom (fluorine, nitrogen, or oxygen ) exists in the vicinity of another electronegative atom with a lone pair of electrons. This type of bond is weaker than an ionic or covalent bond, but it is stronger than van der Waals forces. A simple example of a hydrogen bond is the force of attraction between water molecules.
More Links
HCL Intermolecular Forces
Dipole Moment – Definition, Explanation, and Formula
Combustion Reactions| Introduction, Reaction, & Facts
O2 Molar Mass
Density of Water in g/ml-Accurate Value
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
Absolute Alcohol| Definition, and Formula
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023