A molecule is the smallest particle of a material that possesses all of its physical and chemical features. One or more atoms make form a molecule. If they include more than one atom, the atoms might be the same (for example, an oxygen molecule has two oxygen atoms) or different (a water molecule has two hydrogen atoms and one oxygen atom). Many thousands of atoms may make up biological components such as proteins and DNA.
Table of Contents
Examples of Molecules
The Difference Between a Molecule and a Compound
When two or more atoms combine chemically (can be of the same type or different) they form molecules.
All molecules may be divided into two types: homoatomic and heteroatomic.
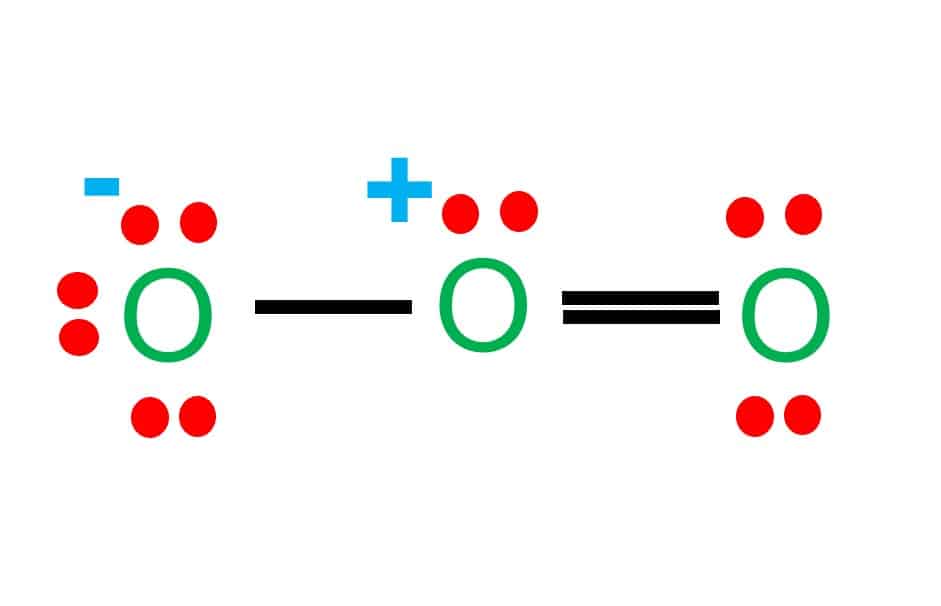
Homoatomic molecules are molecules composed of two or more atoms of the same kind. For example, O2, H2, O3, P4, S8, and so on. Heteroatomic molecules are defined as molecules composed of two or more atoms of different kinds. CO2, H2O, CH4, HCN, SO4, NO, and so on.
Compounds are made up completely of heteroatomic molecules rather than homoatomic ones. When two or more atoms of different kinds combine chemically, a heteroatomic molecule is produced. When these heteroatomic molecules combine, a compound is formed.
Frequently Asked Questions
1. What is a molecule’s simple definition?
A molecule is the smallest component of a material that possesses all of its physical and chemical characteristics. One or more atoms make form a molecule.
2. Is an atom a molecule?
A molecule is made up of atoms that have been linked together. So, whereas an atom is a distinct entity in and of itself, a molecule is formed when those atoms join together.
3. How many atoms make a molecule?
A molecule is a group of two or more atoms joined by chemical bonds. As a result, a molecule must have at least two atoms.
4. What is an atom?
An atom is a matter particle that uniquely characterizes a chemical element. An atom is made up of a core nucleus surrounded by one or more electrons. Each electron carries a negative charge. The nucleus is positively charged and contains one or more protons and neutrons.
5. Is an element a molecule?
An element is a substance composed completely of a single type of atom. For example, the element sodium is composed entirely of sodium atoms. A molecule is a composition of two or more chemically linked atoms, such as the molecule of water and oxygen.
6. ionic compounds?
Ionic compounds are those that are made up of positive and negative ions. Electrons can travel from one atom to another, resulting in the formation of species with varying electric charges.
7. What is hydrogen ion definition?
A hydrogen ion is the nucleus of a hydrogen atom that has been separated from its electron. The hydrogen nucleus is made up of a proton, which is a particle with a unit positive electric charge.
8. How cold is liquid nitrogen?
Liquid nitrogen is simply extremely cold nitrogen. It is 320 degrees Fahrenheit below zero (-196oC). It’s so cold that anything it touches instantly freezes.
Check the full article “How cold is liquid nitrogen?”.
9. ideal gas law
The Ideal Gas Law is a simple equation that shows the connection between temperature, pressure, and volume in the case of gases.
More Links
Cathode| Component of Cells and Batteries
Electromagnet| A Simple Overview
Conduction in Physics| Easy Examples
Convection| Atmospheric Motions in the Vertical Direction
Distillation| Principles, and Processes
The pH of Distilled/ De-Ionized Water
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023