Electron affinity (EA) is defined as the change in energy (kJ/mole) of a neutral atom (in the gaseous phase) when one electron is added to it to create a negative ion. In other words, EA is the likelihood of a neutral atom acquiring an electron.
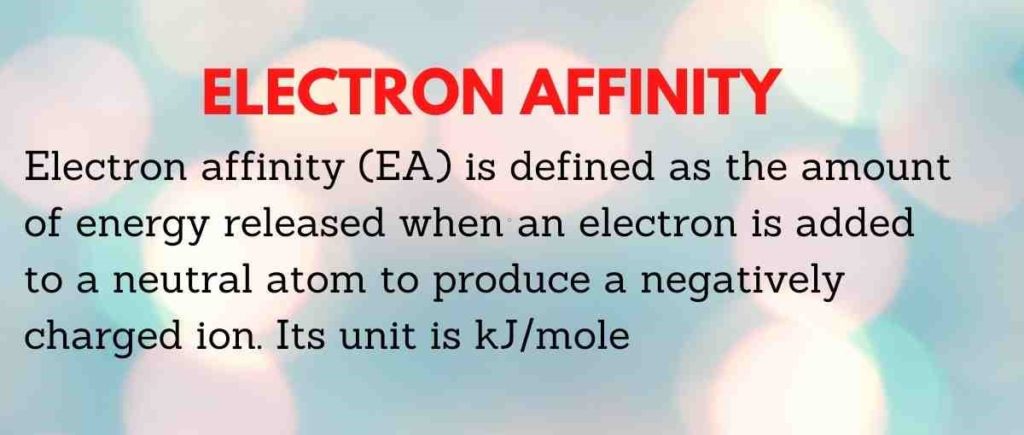
Table of Contents
Electron Affinity In Simple Words
Electron affinity (EA) is defined as the amount of energy released when an electron is added to a neutral atom to produce a negatively charged ion.
Electron Affinity Trend
There are general trends in EA across and down the periodic table of elements.
Along the Period
EA generally increases across a period (row) in the periodic table, due to the filling of the valence shell of the atom.
For example, when a Group-17 atom gains an electron, it releases more energy than a Group-1 atom in that same period because the additional electron forms a full valence shell, which is more stable.
Along the Group
Electron affinity decreases down the groups in the periodic table since the additional electron is entering an orbital farther away from the nucleus. Since this electron is farther away, it should be less attracted to the nucleus and release less energy when added.
Key Points
- Electron affinity increases along the period in the periodic table and decreases down a group.
- The chemical reason for changes in EA across the periodic table is the increased effective nuclear charge across a period and up a group.
- EA is measured for atoms and molecules in the gaseous state only, since in the solid or liquid phases their energy levels would be altered by contact with other atoms or molecules.
- An electron acceptor is a molecule or atom with a higher positive EA value; an electron donor has a lower positive EA value. Charge-transfer reactions may occur when they are combined.
- Electron capture is an exothermic process for almost all non-noble gas atoms because it entails the release of energy.
- The change in energy (E) for any reaction that releases energy has a negative value, and the reaction is referred to as an exothermic process.
Electron affinity vs Electronegativity
An atom’s ability to attract electrons is called electronegativity, and the amount of energy released when an electron is added to a neutral atom is called electron affinity. They follow the same pattern (increase as you go across and decrease as you go down the periodic table).
| fluorine | 328 kJ/mol |
| Oxygen | 141 kJ/mol |
| Chlorine | 349 kJ/mol |
| Lithium | 59.6 kJ/mol |
| Phosphorus | 72 kJ/mol |
| Sulfur | 200 kJ/mol |
| Bromine | 324.6 kJ/mol |
| Magnesium | 0 kJ/mol |
| Neon | 0 kJ/mol |
| Silicon | 133.6 kJ/mol |
Related Links
CO2 Lewis Structure and Molecular Geometry
SiO2 Lewis Structure
SO2 (Sulfur Dioxide) Lewis structure
N2O Lewis Structure| Laughing Gas
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023