A liter of water equals 1000 grams, 35.274 ounces, 0.264 US gallons, or 4.2 US cups (236.59 milliliters) at 17 °C. Furthermore, if we consider the capacity of a glass of water to be equal to 8 ounces, then 1 liter of water is equal to 4.40 glasses (32 ounces).
In addition, it’s important to define a liter here. A liter is a volume unit defined as the volume of one kilogram of pure water. A liter is 1000 milliliters, or roughly 0.03338 gallons or 0.00338 cubic centimeters.
| 1 liter of water | 1000 grams, 35.274 ounces, or 0.264 US gallons |
| 1 US gallon of water | 8.345 pounds or 3.785 kg at 17 °C |
| The specific gravity of water | The specific gravity of water is 1 at 4 degrees Celsius. Specific gravity is a ratio and has no units. |
| Unit weight of water (weight/volume) | 1 g/cm3 at 25 degrees Celsius |
| Cubic Foot of Water Weight | 62.48 pounds at room temperature. 7.5 gallons of water. |
The weight of water depends upon the temperature of the water, For the sake of the question, “How much is a liter of water?”, we are using the density value of water as 0.99802 g/cm3 ( rounded value of 1 g/cm3) at 17 °C. As per the density definition, the weight of the water can be calculated by multiplying the density of water by the volume of water.
1 gram = 0.035274 ounces (oz) = 0.001 kilograms (kg) = 0.00220462 pounds (lb)
| Volume | Weight (g) | Weight (oz) | Weight (kg) | Weight (lb) |
| 1 liter | 1000 g | 35.274 oz | 1 kg | 2.205 lb |
| 1 cup | 236.59 g | 8.345 oz | 0.2366 kg | 0.5216 lb |
| 1 gallon | 3,785.4 g | 133.53 oz | 3.785 kg | 8.345 lb |
| 1 pint | 473.18 g | 16.691 oz | 0.4732 kg | 1.043 lb |
| 1 quart | 946.35 g | 33.382 oz | 0.9464 kg | 2.086 lb |
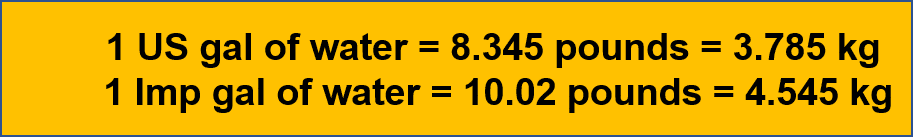
Table of Contents
How much is a liter of water?
At 17 degrees Celsius, the density of water is 0.99802 g/cm3.
Density = mass / volume
Mass = density x volume = 0.99802 g/cm3 x 1000 cm3 = 998.02 grams = 1000 grams (rounded value)
1 gram = 0.035274 ounces (oz).
1 liter of water = 35.274 ounces.
Units of Gallon
There are three different units of a gallon:
| Weight of a Gallon of Water | Fluid ounces | Liters | lbs | Kg |
| 1 US gallon of water at 17 °C | 128 | 3.785 | 8.345 | 3.785 |
| 1 Imperial gallon of water at 17 °C | 153.72 | 4.545 | 10.02 | 4.545 |
| 1 US dry gallon of water at 17 °C | 128 | 4.405 | 9.711 | 4.405 |
How much is a liter?
1 litre (or liter, in the US) = 0.264172 US gallon
1 liter = 1000 ml
1 liter = 1.05669 US quart
1 liter = 4.16667 cups
How many cups are in a liter?
A liter contains 4.227 cups. A cup holds 8 fluid ounces, whereas a liter holds 33.8 fluid ounces. A fluid ounce is a unit of liquid measurement. A British pint has twenty fluid ounces, whereas an American pint contains sixteen.
How many liters of water make a gallon?
1 Gallon [Fluid, US] = 3.7854118 Liters
1 Gallon [Dry, US] = 4.4048838 Liters
1 Gallon [UK] = 4.54609 Liters
Effect of Density on Water Weight
The temperature of the water can affect the density of the water.
Water has a maximum density at 39.2ºF or 4ºC.
At this temperature, a gallon of water weighs around 8.345 lbs.
If we turn the heating way up to 200ºF though, a gallon of water will weigh around 8.04 lbs.
At room temperature (70°F or 21°C), a US gallon of water weighs 8.33 lbs (3.78kg).
The density of water in g/ml is around 1 gram per milliliter at 17 ºC.
How many liters are in cubic centimeters?
1 liter = 1dm3.
1 liter = (10 cm)3 = 1000 cm3 .
1 cm3 = 10-3 liters.
The Effect of Temperature on the Weight of Water
Even moderate fluctuations in temperature do affect the weight of water.
When we heat up water, we increase the energy of water molecules.
As a result, the kinetic energy of water molecules increases, and they get more spaced out.
Therefore, the amount of space each molecule needs is greater.
This results in a decrease in the density of water.
Therefore, the density of the cold water is greater than it would be in the hot water.

Example Problem-Density of Water
The mass of 5 liters of water is 5 kg. Its density will be:
Volume = 1 liter = 10-3 m3
5 liters = 5×10-3 m3.
mass = 5 kg.
density = mass/volume.
The density of water = 5kg/5×10-3 m3 = 1000 kg/m3.
How much water is a mole of water?
One mole of water is 6.022 x 1023 water molecules.
Mass of 1 mole of water = mass of hydrogen + mass of hydrogen + mass of oxygen.
= 1.0079 g + 1.0079 g + 15.9995 g = 18.0152 g
volume = density/mass
density of water is 1 gram per milliliter.
volume = 1 gram per milliliter/18.0152 g = 18ml.
One mole of water equals to18ml of water.
The specific gravity of water
The specific gravity of a material is defined as the ratio of its density to that of water at 4 °C (where it is most dense and is taken to have a value of 999.974 kg m-3). As a result, it is a relative quantity that lacks units. The specific gravity of water is one at 4 degrees Celsius. This is the standard value used by scientists to determine the specific gravity of various substances.
Important Links
Related Links
How Many Bottles Of Water Equal A Gallon
Concentration Gradient Definition
How Much Does a Gallon of Water Weigh?
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
How Many Water Bottles is a Gallon| Examples
Frequently Asked Questions
Some of the frequently asked questions are given below.
1. How many glasses are in one liter of water?
If we consider the capacity of a glass of water to be equal to 8 ounces, then 1 liter is equal to 32 ounces.
2. What is Freshwater?
Freshwater is naturally occurring water with less than 500 parts per million (ppm) of dissolved salts. Generally, it is called “salt-free water.” It is safe to drink. Only three percent of the earth’s water is freshwater. Its habitats are less than 1% of the world’s total surface area.
Its recommended basic requirement for human needs (per person) is 5 liters/day.
3. Why does ice float on water?
Solid water (ice) is less dense than liquid water.
Every water molecule is made up of two hydrogen atoms bonded to one oxygen atom.
Due to its molecular structure, water behaves abnormally when cooled.
It continues to contract and reduce in volume until 4°C.
Water has a maximum density at 39.2ºF or 4ºC.
Beyond this point, its volume increases and becomes more than the initial volume.
This results in a decrease in density. That’s why ice is lighter than liquid water.
4. What is a water molecule and how many hydrogen atoms are in a molecule of water?
Water is a substance that exists in gaseous, liquid, or solid phases and is made up of the chemical elements hydrogen and oxygen. It is one of the most abundant and necessary chemicals. At room temperature, it is a tasteless and odorless liquid with the critical capacity to dissolve many other compounds. Water’s capacity as a solvent is critical to living creatures.
A water molecule consists of two hydrogen atoms attached to the sides of a single oxygen atom.
5. What is the specific heat formula?
Specific heat formula is the amount of heat supplied to the specimen, divided by the resulting temperature increase. cp is related to the unit mass of the specimen. The mathematical form of the formula is Q/m ΔT = cp where Q refers to the amount of heat supplied to the specimen and ΔT is the temperature rise.
6. What is the weight of water in kg?
At 25°C (77°F or 298.15K), the weight of water is1 gram per cubic centimeter, or 1,000 kilograms per cubic meter. This means that the density of water is 1 000 kg/m3. Weight per cubic foot: 62.4 pounds per cubic foot [lb/ft3], or 0.58 ounces per cubic inch [oz/inch3].
7. What is the density of water in lbft3?
Density is a physical property that expresses the mass per unit volume. In the case of liquid water, the density of water is close to 1000 kg/m3, though it varies slightly depending on temperature. The density of water lb/ft3 is 62.428 lb/ft3.
8. How many water bottles are in a liter?
A liter of water contains two bottles of water with each bottle of 500 ml capacity.
More Interesting Topics
Author
Written by Umair Javed, MS.
Umair has been working at Whatsinsight since 2020 as a content writer.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



