Cathodes are essential components of batteries, fuel cells, and other polarized electrical devices like vacuum tubes. In an electrochemical cell, the cathode is the positive or oxidizing electrode that accepts electrons from the external circuit and reduces itself during the electrochemical process. In electrical devices, electrons always flow from the anode to the cathode.
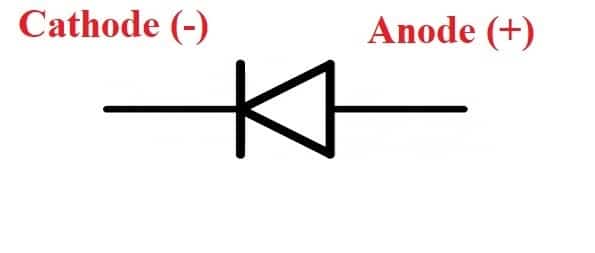
In an electrolytic cell, the battery moves electrons away from the anode (making it positive) and into the cathode (making it negative). The positive anode draws anions toward it, whereas the negative cathode attracts cations toward it.
Table of Contents
The cathode is positive in Galvanic cell and negative in Electrolytic cell
The anode of a galvanic (voltaic) cell is regarded as negative, whereas the cathode is considered positive. This sounds reasonable given that the anode is the source of electrons and the cathode is the destination of electrons.
In an electrolytic cell, however, the anode is considered to be positive while the cathode is considered to be negative. However, the reaction remains the same, with electrons from the anode flowing to the battery’s positive terminal and electrons from the battery flowing to the cathode.
Anode vs Cathode
| Anode | Cathode |
| The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. | The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. |
| The anode is regarded as negative in a galvanic (voltaic) cell | The cathode is deemed positive in a galvanic cell. |
| In an electrolytic cell, it is a source of positive charge or an electron acceptor. | In an electrolytic cell, it is a source of negative charge or electron donor. |
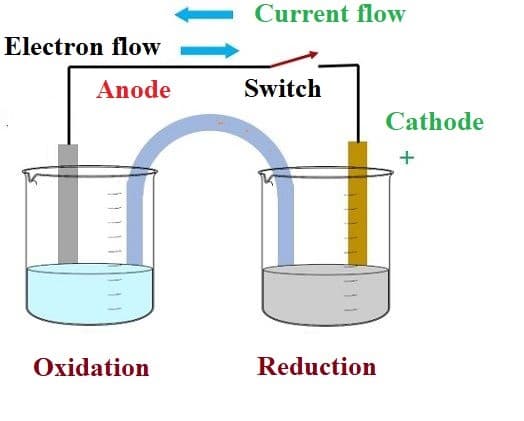
Galvanic Cell
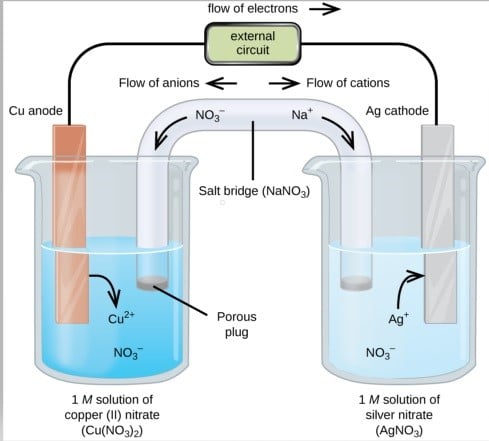
A galvanic cell is an electrochemical cell that turns a chemical process’s free energy into electrical energy. A galvanic cell is composed of two halves, anodic and cathodic. The cell reaction is redox in nature. The anode is responsible for oxidation, whereas the cathode is responsible for the reduction. Electrons always flow from anode to cathode or from oxidation half cell to reduction half cell.
Summary
- An electrochemical cell is a device or process that may create electrical energy from a chemical reaction or use electrical energy to generate a chemical reaction.
- Electrochemical cells are classified into two categories based on the processes that occur within them: galvanic or voltaic cells and electrolytic cells. Electrolytic cells turn electrical energy into chemical energy, whereas galvanic cells convert chemical energy into electrical energy.
- The electrochemical cell is made up of four major components: The anode is the unit where oxidation takes place. The cathode is the unit where reduction takes place. Electrons can flow through an external channel. A salt bridge or porous barrier allows ions to travel back and forth, preventing charge buildup.
- Reduction always takes place at the cathode, while oxidation always takes place at the anode. Because reduction involves the addition of electrons, electrons must go toward the point of reduction. The negative charge is on the cathode of an electrolytic cell, whereas the positive charge is on the anode.
Frequently Asked Questions
1. What is cathode definition?
The cathode is the electrode of an electrochemical cell where reduction takes place. In layman’s terms, the cathode is an electrolytic cell’s negative terminal.
2. What is a cathode ray tube?
A cathode-ray tube is a type of vacuum tube that produces pictures when an electron beam impacts a phosphorescent surface. Cathode ray tubes are used in the majority of desktop computer screens. A computer display’s CRT is analogous to a television receiver’s “picture tube”.
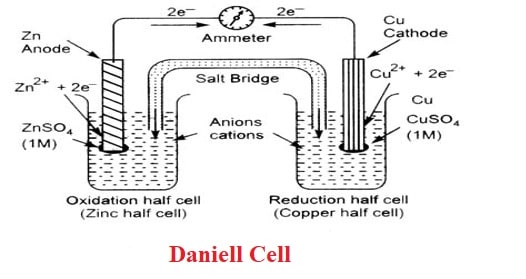
3. What is Daniell Cell?
A Daniell cell is a form of a galvanic cell that transfers chemical energy to electrical energy. The cell is made up of zinc and copper electrodes, with zinc functioning as the anode and copper acting as the cathode. Electrons always move from the anode to the cathode or from the oxidation half cell to the reduction half cell. The Daniell Cell has a voltage of 1.1 volts.
4. Is helium a gas?
Helium (He) is an inert gas and chemical element in Periodic Group 18. (noble gases). Helium, the second lightest element (only hydrogen is lighter), is a colorless, odorless, and tasteless gas that freezes at 268.9 degrees Celsius (452 degrees Fahrenheit).
5. At what temperature does water freeze?
Water’s normal freezing and melting points are 0 degrees Celsius or 32 degrees Fahrenheit.
6. Hydrogen cyanide polar or nonpolar?
HCN is a polar molecule.
As can be seen from the HCN lewis structure, the electronegativity difference between nitrogen (3.04) and hydrogen (2.2) makes it a polar molecule.
7. What is the melting point of water?
The melting point of pure water ice at 1 atmosphere of pressure is very close to 0 °C, which is 32 °F or 273.15 K.
8. What is the surface tension of water?
Surface tension refers to the ability of a liquid’s surface to resist an external force because of its molecules’ cohesion. At 20 °C (68 °F), the surface tension of water is 0.07275 joules per square meter.
9. Is MgCl2 Ionic or Covalent?
When the magnesium atom loses two electrons to create the Mg2+ ion and each chlorine receives one electron to form the Cl– ion, an ionic connection is formed between the magnesium and chlorine atoms.
Check “Is MgCl2 ionic or covalent?”.
10. What is the hydrogen phosphate formula?
The hydrogen phosphate formula is [HPO4]2-. The molar mass of this compound is 95.97 g/mol. The ion is formed by the loss of two protons H+ from phosphoric acid H3PO4, which accounts for the ion’s charge of 2.
11. What is a hydrogen ion?
The nucleus of a hydrogen atom separated from its electron is known as a hydrogen ion. The hydrogen nucleus is made up of a proton, which is a particle with a unit positive electric charge.
12. Is hydrogen sulfide poisonous?
Hydrogen sulfide (H2S) is made up of a single sulfur atom bonded to two hydrogen atoms. It is a highly poisonous, flammable gas with the odor of rotten eggs that are frequently produced by bacterial decomposition of organic matter in the absence of oxygen.
13. What is the electronegativity value of hydrogen?
The electronegativity of hydrogen is 2.2.
Electronegativity is used to determine whether an ionic or covalent connection will form between two atoms. It can also determine whether the molecule is polar or nonpolar.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023