Acetylene (also called Ethyne) is the most basic of the alkyne hydrocarbons, which have triple bonds between two carbon atoms. Acetylene is employed in the production of various chemicals and as a welding fuel, with an acetylene oxygen flame capable of reaching 3200 °C.
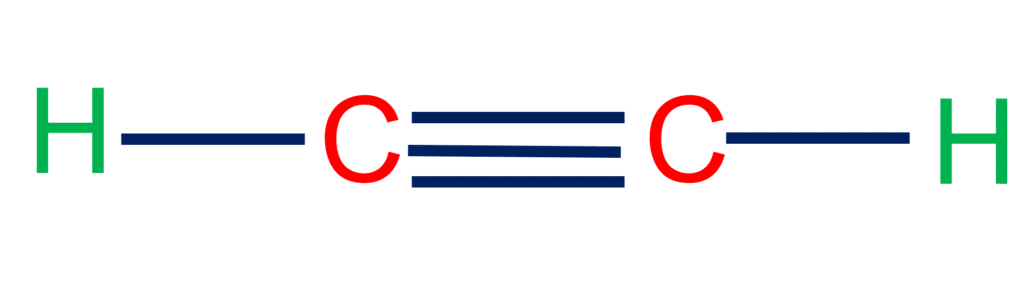
In acetylene, the carbon atoms are held together by a triple bond, a sigma bond, and to pi bond. The electron density between the carbon atoms is very high, which draws atoms very close to each other. Electrons in a triple bond are, therefore, less exposed and thus less reactive towards electrophilic reagents.
| Compound Name | Acetylene |
| Other names | Ethyne, Narcylen, Vinylene, Ethine, Ethylene |
| Appearance | Colorless gas with a sweet taste |
| Boiling point | -84 Celsius degree |
| Molecular weight | 26.04 |
| Density | 1.1 kg/cubic meter |
| Solubility | soluble in water and other organic compounds and slightly soluble in acetic acid and ethanol. |
Table of Contents
Combustion of Acetylene?
When acetylene is burned in air or oxygen, it produces heat and emits CO2 and H2O. The reaction is extremely exothermic, producing an oxyacetylene flame that is employed in metal welding and cutting.
2 H2C2 (g) + 5 O2 (g) –> 4 CO2 (g) + 2 H2O (g)
Sigma and Pi Bonds in Acetylene
There are three sigma bonds and two pi bonds in acetylene.
Acetylene contains three sigma bonds, two of which are between the hydrogen and carbon atoms and one between the two carbon atoms. The other two bonds are pi-bonds, which are formed by the overlap of two pure p orbitals.
Uses of Acetylene
Approximately 80% of the acetylene produced is utilized in chemical synthesis.
Acetylene is an important raw material for a variety of organic compounds such as acetaldehyde, acetic acid, acetic anhydride, acetone, and vinyl chloride, which are used to make a wide range of products such as plastics, synthetic rubber, dyestuffs, solvents, and pharmaceuticals, as well as carbon black.
The remaining 20% is utilized for oxy-acetylene welding, cutting, heat treatment, illumination, buoys, and beacons.
Related Links
Combustion Reactions| Introduction, Reaction, & Facts
Hydrogen Molar Mass
Acidic Hydrogen
HCl Intermolecular Forces| Dipole-Dipole Forces
Molar Mass of Methanol| Easy-Explanation
Is HCl Polar or Nonpolar?
Frequently Asked Questions
1. Is chlorine a metal?
Chlorine (Cl) is the second lightest member of the periodic table’s halogen elements, or Group 17 (Group VIIa). Because it lacks metal-like properties such as electrical conductivity, flexibility, and strength, chlorine is classified as a nonmetal.
Check the full article “Is chlorine a metal?”.
2. Is hydrogen a metal?
While hydrogen has the same ns1 electron configuration as alkali metals, it is not considered a metal because it forms cations (H+) more slowly than other alkali metals. As a result, the simple answer to the question “is hydrogen a metal?” is no.
3. What is magnesium chloride?
Magnesium Chloride (MgCl2) is an ionic chemical and magnesium source that is used to replace electrolytes and cure magnesium shortage diseases. Magnesium Chloride anhydrous contains 25.5 percent elemental magnesium by mass.
4. What is sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
5. What is Zinc Hydroxide?
Zinc hydroxide is an inorganic chemical compound with an amphoteric character and may dissolve in both a strong basic and an acid solution. It is frequently used as a galvanic material to prevent metal substrate corrosion. The zinc hydroxide formula is Zn(OH)2.
More Links
Is O2 Polar or Nonpolar?
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
How Many Neutrons Does Hydrogen Have?
CH4 Polarity| Simple Explanation
Charge of Ammonia (NH3)| Simple Steps
HCl Intermolecular Forces| Dipole-Dipole Forces
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



