Styrene (also known as styrene monomer, vinyl benzene, phenylethylene, styrol, styrole, styrolene, cinnamene, styron, and cinnamol) is a clear, colorless to yellow oily liquid with a sweet odor. It is an important component in the manufacture of thousands of everyday products because it provides strength and flexibility while remaining lightweight.
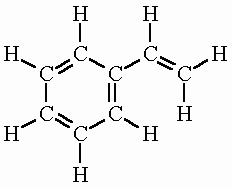
Styrene has the formula C8H8 and is combustible as well as readily evaporating.
Styrene is used in the production of latex, synthetic rubber, and polystyrene resins. These resins are used in the production of plastic packaging, disposable cups and containers, insulation, and a variety of other items.
| Compound Name | Styrene (C8H8) |
| Appearance | Clear, colorless to the yellow oily liquid |
| Boiling point | 145 °C |
| Molar Mass | 104.15 g/mol |
| Density | 909 kg/m³ |
| Solubility | Soluble in water, ethanol, benzene, and petroleum products. |
| Uses | Used in the production of plastic packaging, disposable cups and containers, insulation, and a variety of other items. |
| Chemical Safety | Flammable, Irritant, Health Hazard |
Styrene can be produced via a variety of methods. One of these processes is the dehydrogenation of ethylbenzene with iron as a catalyst. This technique accounts for 85 percent of global styrene production.
Table of Contents
What is Styrene (C8H8)?
Styrene is a chemical that is used in the production of latex, synthetic rubber, and polystyrene resins. These resins are used in the production of plastic packaging, disposable cups and containers, insulation, and a variety of other items. Styrene is also naturally generated by several plants.
The molecular structure of styrene is an aromatic ring of 6 members with a vinyl group -CH=CH2 bond to the position carbon. The styrene molecule contains a total of 16 bond(s) There are 8 non-H bond(s), 7 multiple bond(s), 1 rotatable bond(s), 1 double bond(s), 6 aromatic bond(s) and 1 six-membered ring(s).
Related Links
Combustion Reactions| Introduction, Reaction, & Facts
Hydrogen Molar Mass
Acidic Hydrogen
C6H12O6- 10 Key Points- Easy Explanation
What is Carbon Footprint?| Definition and Keypoints
How cold is Liquid Nitrogen?
Frequently Asked Questions
1. Is chlorine a metal?
Chlorine (Cl) is the second lightest member of the periodic table’s halogen elements, or Group 17 (Group VIIa). Because it lacks metal-like properties such as electrical conductivity, flexibility, and strength, chlorine is classified as a nonmetal.
Check the full article “Is chlorine a metal?”.
2. Is hydrogen a metal?
While hydrogen has the same ns1 electron configuration as alkali metals, it is not considered a metal because it forms cations (H+) more slowly than other alkali metals. As a result, the simple answer to the question “is hydrogen a metal?” is no.
3. What is magnesium chloride?
Magnesium Chloride (MgCl2) is an ionic chemical and magnesium source that is used to replace electrolytes and cure magnesium shortage diseases. Magnesium Chloride anhydrous contains 25.5 percent elemental magnesium by mass.
4. What is sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
5. What is Zinc Hydroxide?
Zinc hydroxide is an inorganic chemical compound with an amphoteric character and may dissolve in both a strong basic and an acid solution. It is frequently used as a galvanic material to prevent metal substrate corrosion. The zinc hydroxide formula is Zn(OH)2.
More Links
Is Water a Mixture?
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
How Many Neutrons Does Hydrogen Have?
Viscosity of Water
Liquefied Natural Gas (LNG)| Short Overview
Liquefied Petroleum Gas| Introduction, and Uses
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



