The Diffusion coefficient (D), also known as mass diffusivity, is a measurement of how quickly one material diffuses through another. Diffusion will be faster if the D is higher. Many equations, notably Fick’s First and Second Laws, include it as a key variable.
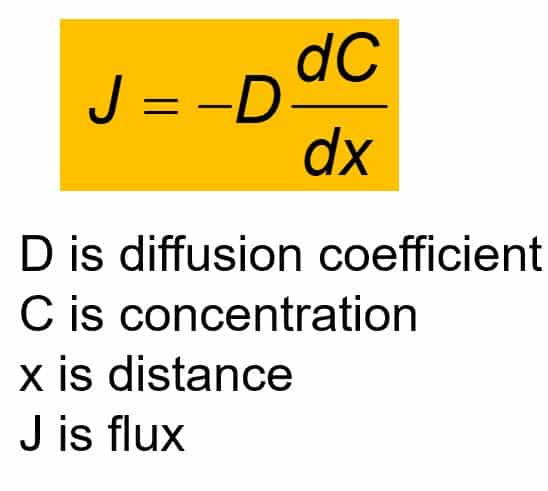
The above equation (Fick’s first law) indicates that if the flux and the change in the concentration over time are known, then the D can be calculated. The negative sign indicates that the concentration gradient is negative.
Table of Contents
Diffusion Coefficient
Diffusion coefficient (D) is the proportionality factor in Fick’s Law.
Fick’s law states that the mass of a substance diffusing in time over a surface normal to the diffusion direction is proportionate to its concentration gradient.
Diffusion Coefficient Units
The diffusion coefficient (mass diffusivity) has units of area per time (m2/s) in SI units. The term refers to the mass of substance, with a concentration gradient of unity, diffuses through a unit surface in a unit of time.
What is Rate of Diffusion?
The rate of diffusion or flux (J) is defined as the amount of substance that will flow through a unit area during a unit time interval.
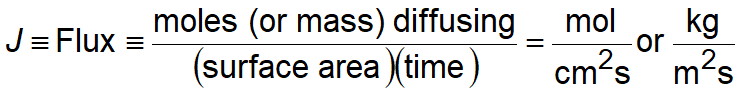
Arrhenius Relationship
The Arrhenius equation is a formula for the temperature dependence of reaction rates in physical chemistry. It can be used to simulate temperature-dependent diffusion coefficients, crystal vacancy populations, creep rates, and a variety of other thermally-induced processes and reactions.
To describe the diffusivity using the Arrhenius relationship, Consider an atom diffusing through a crystalline solid: it has to move through the lattice.
There can be two ways to diffuse:
- Solute atom small enough to fit into the empty spaces between the atoms of the solvent lattice
- Another way is vacancy diffusion in which atoms exchange with vacancies. This diffusion applies to substitutional impurities atoms. Its rate depends upon the number of vacancies and activation energy to exchange.
The Arrhenius equation given below shows that mass diffusivity (D) increases with temperature.
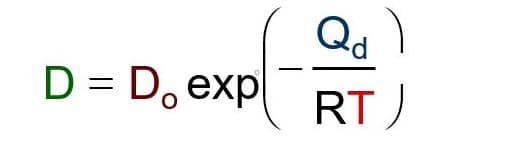
D= Diffusion coefficient [m2/s]
Do = Pre-exponential [m2/s]
Q= Activation energy [J/mol or eV/atom]
R= Gas constant [8.314 J/mol-K]
T= Absolute temperature [K]
Factors Affecting Diffusion Coefficient
The diffusion coefficient depends on the specific circumstances the diffusion is occurring in, including what materials are involved and the state of the surrounding environment. Some of the factors are:
- Molecule size of diffusing material
- The temperature of the system and surrounding
- Pressure
- Solvent viscosity
- Solution properties as salt concentration
Sample Problem
Paint removers frequently contain methylene chloride. It is an irritant that can also be absorbed by the skin. Protective gloves should be worn when using this paint remover. If butyl rubber gloves (0.04 cm thick) are used, find the diffusive flux of methylene chloride.
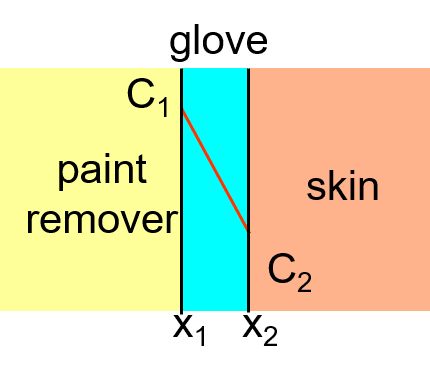
D = 110 x10-8 cm2/s
surface concentrations are:
C1 = 0.44 g/cm3
C2 = 0.02 g/cm3
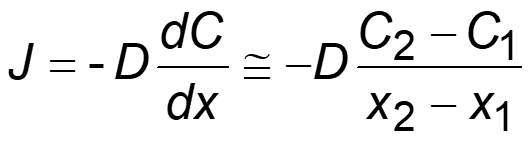
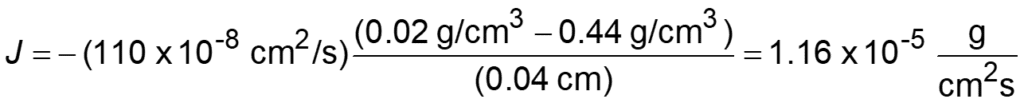
Diffusion
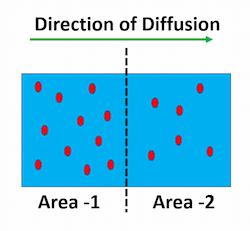
The net transport of molecules from a higher concentration area to a lower concentration area is known as diffusion. This is owing to the spontaneous movement of the molecules.
In other words, Diffusion is the net movement of anything (for example, atoms, ions, molecules, and energy) from a higher to a lower concentration region. Some examples are listed below:
- The aroma of food diffuses into the air and the smell reaches you.
- A tea bag placed in a cup of hot water will diffuse into the water.
- The smell of perfume spreads over a whole room is an example of diffusion
- Steel can be diffused (e.g., with carbon or nitrogen) to modify its properties.
Benefits of Diffusion
- Diffusion is a process that aids in the movement of molecules into and out of cells. The molecules travel from a high-concentration zone to a low-concentration region until the concentration is uniform throughout.
- It is an important phenomenon that occurs in a variety of biological activities like the net movement of particles, ions, molecules, solutions, and so on.
- It is a crucial factor in the movement of molecules during the metabolic process in all living organisms.
Factor Affecting Diffusion
Diffusion is a natural and physical process that occurs without the need to mix or shake the solutions. Diffusion occurs in liquids and gases because molecules can flow at random. The molecules collide and shift the direction of the flow. Some of the factors affecting this process are listed below:
- Temperature
- Area of Interaction
- Size of the Particle
- The difference in concentration
Dynamic Viscosity
The resistance to the flow of one layer of fluid across another layer of fluid is defined as dynamic viscosity. Viscosity is caused by friction inside a fluid. The intermolecular forces that exist between particles in a fluid cause it.
Related Links
Condensation Reaction| Easy Explanation
Dynamic Viscosity-An Overview
Heat Flux-An Overview
Momentum Equation| Definition and Examples
How Much is a Liter of Water?
Frequently Asked Questions (FAQs)
1. What is the difference between steady-state diffusion and unsteady state diffusion?
There are two forms of diffusion: steady-state diffusion and unstable state diffusion. The major distinction between steady-state and unstable state diffusion is that steady-state diffusion occurs at a constant rate, whereas unsteady-state diffusion occurs at a rate that varies with time. Fick’s laws can quantitatively characterize both of these categories.
2. What is the concentration gradient definition in biological terms?
A concentration gradient occurs when the concentration of something builds upon only one side of a membrane.
Organisms that need to move material into or out of their cells may use force driven by differences in concentration. Protein antiporters and symporters use this basic technique to bring essential nutrients into cells.
3. Define nonsteady state diffusion?
The rate of diffusion in unsteady-state diffusion, also known as non-steady state diffusion, is a function of time. This means that the pace of diffusion varies throughout time. As a result, the concentration rate with distance (dc/dx) is not constant, and the concentration change with time is not zero.
4. What is laminar flow?
Laminar flow is described in fluid dynamics as the smooth or regular movement of fluid particles, as opposed to turbulent flow, which is defined as the irregular movement of fluid particles. Because the fluid travels in parallel layers, there is no break between them (with limited lateral mixing).
Important Links
Mechanical Energy Formula & Examples
Room Temperature| Comfortable Temperature
Negative Kinetic Energy| 6-Easy Examples
How Many Cups in a Gallon? Cups to Pints, Quarts
Density of Water in g/ml-Accurate Value
The Density of Water lbs/U.S gal
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023