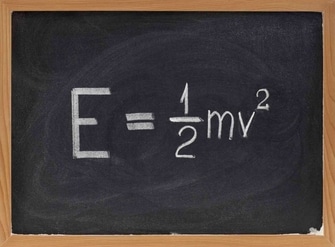The thermal energy of an object is the energy contained in the motion and vibration of its molecules. It is the kinetic energy associated with the random movements of a material’s molecules; the phrases “heat” and “heat energy” are sometimes used interchangeably. The SI unit of thermal energy is joules (J). Other units like calories and Btu are also used as well.
In simple words, the thermal energy equation shows the energy that comes from the temperature of matter. The hotter the substance, the greater the vibration of molecules and hence the higher the thermal energy.
The thermal energy equation is given as Q=mcΔT.
where Q is the symbol for heat transfer, m is the mass of the substance, and ΔT is the change in temperature. The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature of 1 kg of mass by 1.00ºC.

Table of Contents
Thermal Energy Equation
change in thermal energy = mass × specific heat capacity × change in temperature
Q = mcΔT
Q: heat energy or thermal energy
m: mass
c: specific heat capacity
ΔT: change in temperature
Examples of Thermal Energy
Here are some examples of thermal energy that you are likely to be familiar with:
- Geothermal Energy
- Heat Energy From the Oceans
- Fuel Cell Energy
Heat Flux
Heat flux (Ф) can be defined as the rate of heat energy transfer through a given surface (W), and heat flux density (φ) is the heat flux per unit area (Wm²).
Thermal Conductivity
Thermal conductivity is defined as the ability of a material to conduct or transfer heat energy. It is generally denoted by the symbol ‘k’ but can also be denoted by ‘λ’ and ‘κ’. The reciprocal of thermal conductivity is known as thermal resistivity. Materials with high thermal conductivity are used in heat sinks, whereas materials with low values of λ are used as thermal insulators.
Quick Links
Thermal Conductivity of Water
The Specific Gravity of Water
The Melting Point of Water
Specific Heat of Water
Energy-The Ability to do Work
Frequently Asked Questions
1. Heat capacity of water?
Heat capacity is the amount of heat energy required to raise the temperature of a given mass of a material by one unit. Water has a relatively high specific heat capacity of 4200 J kg -1 K-1, while copper has a specific heat capacity of 400 J kg -1 K -1.
2. Thermal energy definition?
Thermal energy is the energy that comes from the temperature of matter. The hotter the substance, the greater the vibration of molecules and hence the higher the thermal energy.
3. kinetic energy of water molecules?
The greater the measured temperature, the more kinetic energy a molecule possesses as it travels quicker. The average speed of the water molecules in water at ambient temperature (20 °C or 68 °F) is around 590 m/s (1300 mph). However, this is simply the water molecules’ average (or mean) speed.
4. Is thermal energy potential or kinetic?
Thermal energy is simply another name for heat energy. It is both potential and kinetic energy.
Thermal potential energy is potential energy at the atomic and molecular levels, where it may be converted to kinetic energy or other types of energy. Chemical bonds, electrostatic or intermolecular interactions, and nuclear bonds are all examples of thermal potential energy.
Pistons in a steam engine extract thermal energy from hot gases. The steam engine then converts thermal energy into kinetic energy by using these rotating pistons to power whatever it is connected to.
More Interesting Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023