According to Graham’s law, a gas’s rate of diffusion or effusion is inversely proportional to its molecular weight squared. Diffusion is the spread of one gas into or through another, whereas effusion is the passage of a gas through a small hole.
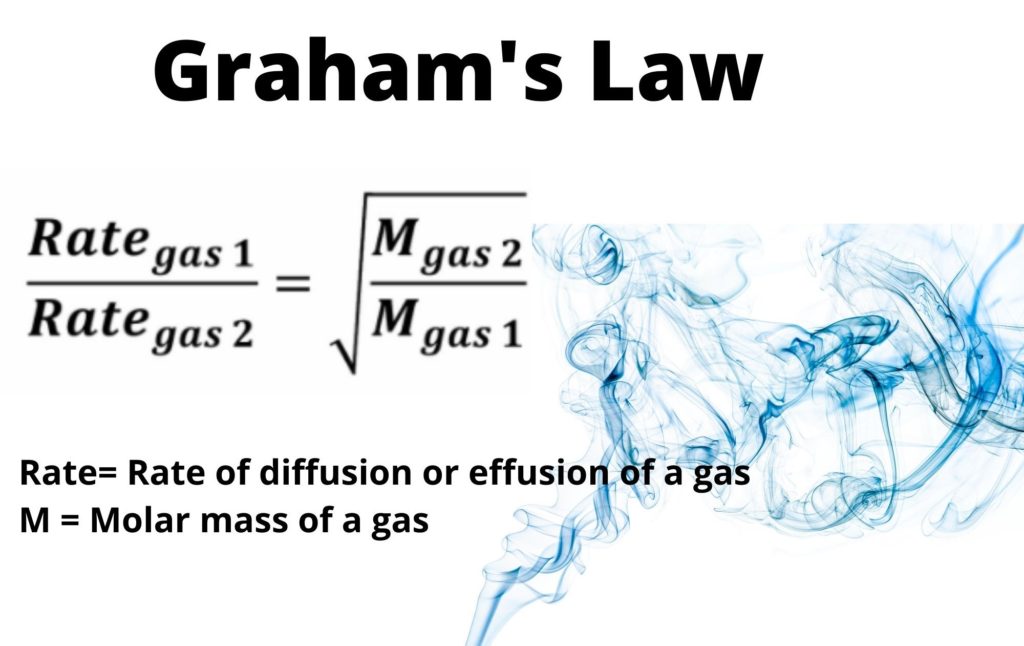
Thomas Graham’s (1805-1869) finding on gas diffusion resulted in what is now known as Graham’s law of diffusion. The law states that the rate of diffusion (or effusion) is directly proportional to the volume of gas diffused (or effused) in a given time and that the density of a gas is proportional to its molar mass led to this formulation. The equation states that the diffusion rate of the first gas over the diffusion rate of the second gas is equal to the square root of the molecular weight of the second gas over the molecular weight of the first gas.
Graham’s Law Formula in Simple Words
Graham’s law formula in simple words states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molecular weight, which is represented by the variable M. This means that the rate of diffusion or effusion of a gas can be calculated using the following formula:
Rate of diffusion/effusion = (square root of M2) / (square root of M1)
Where M1 and M2 are the molecular weights of the two gases being compared. This formula is important in fields such as chemistry and physics, where the diffusion or effusion of gases is a key factor in many processes.
Important Points
| Definition | Graham’s law states that the rates of diffusion or effusion of gases are inversely proportional to the square roots of their molecular weights, when other factors are held constant. |
| Formula | Rate of diffusion/effusion = (square root of M2) / (square root of M1), where M1 and M2 are the molecular weights of the two gases being compared. |
| Unit | The rate of diffusion/effusion is typically expressed in units of cm/s or m/s. |
| Assumptions | The law assumes that the gases being compared are at the same temperature and pressure. The law only applies to ideal gases, which are non-interacting particles that occupy no volume. The law can be used to predict the relative rates of diffusion or effusion of two gases, but it does not provide information about the actual rates of diffusion or effusion. |
| Daily Life Examples | Examples of Graham’s law in daily life include the diffusion of perfume, the rate of air exchange in the lungs during respiration, and the effusion of gas from a helium balloon. |
Graham’s Law Application
- According to the law, gases with heavy molecules travel slower than gases with light molecules. As a result, a distinction between the molecular weights of the two gases is established.
- Graham’s Law determines what we smell more quickly. Some gases travel faster, while others travel slower.
- Graham’s Law governs all living things’ respiration, whether through skin or lungs.
- The diffusion and effusion rates of the gases can be estimated in advance using Graham’s law. This aids in determining the proper height of chimneys required to ensure the escape of smoke and toxic gas particles away from the household and human contact.
- The law is useful for calculating the hardness of the water. If we know the diffusion rate of two water samples, we can use Graham’s law to determine which of the water samples is heavier, thus distinguishing hard water from soft water.
Summary
- The rate of diffusion or effusion of a gas is inversely related to its molecular weight squared.
- The distance traveled by gas molecules per unit of time is known as the rate of diffusion (r) of the gas.
- Spraying Air fresheners and the Separation of gases on the basis of different densities are examples of graham’s law of diffusion.
Multiple Choice Questions
- What does Graham’s Law state?
A) The rates of diffusion or effusion of gases are directly proportional to their molecular weights.
B) The rates of diffusion or effusion of gases are inversely proportional to the square roots of their molecular weights.
C) The rates of diffusion or effusion of gases are directly proportional to the square roots of their molecular weights.
D) The rates of diffusion or effusion of gases are inversely proportional to their molecular weights.
Answer: B
- What is the formula for Graham’s Law?
A) Rate of diffusion/effusion = (M1 + M2) / (M2 – M1)
B) Rate of diffusion/effusion = (M1 – M2) / (M1 + M2)
C) Rate of diffusion/effusion = (M2 – M1) / (M1 x M2)
D) Rate of diffusion/effusion = (square root of M2) / (square root of M1)
Answer: D
- Which unit is typically used to express the rate of diffusion or effusion in Graham’s Law?
A) kg/s
B) g/cm^3
C) m/s
D) J/mol
Answer: C
- Which of the following assumptions is made in Graham’s Law?
A) The gases being compared are at different temperatures.
B) The law applies to all gases, including real gases.
C) The law provides information about the actual rates of diffusion or effusion.
D) The gases being compared are at the same temperature and pressure.
Answer: D
- What is an example of Graham’s Law in daily life?
A) The expansion of a balloon when heated.
B) The behavior of a gas at high pressures and low temperatures.
C) The process of combustion in an engine.
D) The diffusion of perfume in a room.
Answer: D
Solved Numerical Problems
- A gas mixture contains two gases, A and B. Gas A has a molecular weight of 20 g/mol and gas B has a molecular weight of 30 g/mol. Which gas will diffuse faster through a small hole in a container, assuming that the gases have the same temperature and pressure?
Answer: Gas A will diffuse faster through the small hole in a container. Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molecular weight. Since gas A has a lower molecular weight than gas B, it will effuse or diffuse faster through the small hole in the container.
- A gas mixture contains hydrogen gas (H2) and oxygen gas (O2) in a 2:1 volume ratio. If the rate of effusion of hydrogen gas is 5.6 mL/min, what is the rate of effusion of oxygen gas?
Answer: The rate of effusion of oxygen gas is 2.8 mL/min. Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molecular weight. Since the molecular weight of oxygen gas is twice that of hydrogen gas, the rate of effusion of oxygen gas will be half that of hydrogen gas. Therefore, the rate of effusion of oxygen gas can be calculated as:
rate of effusion of oxygen gas = (rate of effusion of hydrogen gas) / square root of 2 rate of effusion of oxygen gas = 5.6 mL/min / 1.414 rate of effusion of oxygen gas = 2.8 mL/min
Frequently Asked Questions
1. What does Graham’s law state?
Graham’s law says that the rate of diffusion or effusion of a gas is inversely related to its molecular weight squared.
2. What is Graham’s Law’s simple definition?
In simple terms, Graham’s law says that a gas’s rate of diffusion or effusion is inversely related to the square root of its molecular weight.
3. What is an example of Graham’s law?
- Spraying Air Freshener
- Separation of gases as different gases having different densities
- A drop of food coloring diffuses throughout the water in a glass so that, eventually, the entire glass will be colored.
4. What is the rate of diffusion?
The distance traveled by gas molecules per unit of time is known as the rate of diffusion (r) of the gas.
5. Ideal gas law in simple words?
The ideal gas law describes how the macroscopic properties of ideal gases are related. An ideal gas is one in which the particles (a) do not attract or repel one another and (b) do not take up any space (have no volume).
More Interesting Topics
Concentration Gradient Definition| Easy Examples
Is Air a Homogeneous Mixture?
Diffusion Coefficient| Mass Diffusivity
Dynamic Viscosity-An Overview
Hess’s Law: Statement, Equation, & Examples
Velocity Formula & Definition
The Density of Water lbs/U.S gal
Density of Water in g/ml-Accurate Value
Momentum Equation| Definition and Examples
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023