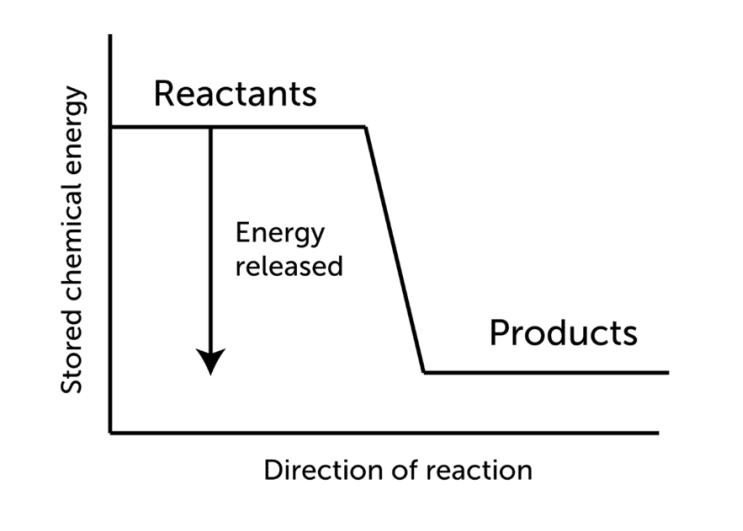
Thermite welding is the technique of joining two or more workpieces by pouring molten metal between them. This process was developed in 1895 and still has its uses on railroads today. It is also known as the Goldschmidt process or exothermic welding.
| Technique | Thermite welding |
| Thermite meaning | A mixture of aluminum powder and metal oxide (such as iron oxide) that, when ignited, produces a large amount of heat and is used in welding. |
| Advantages | When compared to other types of weld, the produced weld has great mechanical strength and good corrosion resistance. |
| Drawbacks | Uneconomical for welding cheap metals and light parts. |
| Uses | Used to fix railroad issues. |
Table of Contents
Working Principle Of Thermite Welding
Thermite welding (TW) is a fusion welding method that bonds two metals after they are heated by superheated metal that has undergone an aluminothermic reaction.
This technique employs heat from an exothermic reaction to create metal coalescence. For instance, to join two steel rails, we warm the steel rails to 600 degrees after setting up the thermite welding equipment. Then, place the welding portion into the crucible and fire it. This will result in molten steel and slag. The high-temperature steel is poured in the contact between two steel rails. According to the reaction’s stoichiometry, the weight ratio of Fe2O3 to Aluminum powder is around 3 to 1. (2.96 to 1 to be more exact).
Thermite Reaction
Aluminum metal is a very strong reducing agent. When heated, it converts metal oxides such as iron and chromium to a metallic state. Thermit reaction is the name given to this reaction. In this reaction, aluminum is used as a catalyst. The balanced chemical reaction between Iron(III) Oxide and Aluminum is shown below,
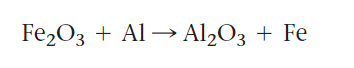
The iron obtained in this manner is molten.
This method is utilized in the welding of railway rails, heavy machinery, and other similar applications.
Keywords- Thermite Welding
- A thermite reaction is also known as the Goldschmidt thermite process or aluminothermy.
- The reaction generates a huge quantity of heat, enough to raise the temperature to over 3500oC.
- Thermite is difficult to ignite.
- The activation energy required to start the reaction using thermite is extremely high.
- Magnesium ribbon may easily ignite thermite when heated to several thousand degrees.
- The magnesium ribbon is important because it works like a fuse, burning gently and enabling a brief wait between when the ribbon is lighted and when the thermite begins to react.
Types of Thermite
- Iron(III) Oxide – Fe2O3
- Iron(II, III) Oxide – Fe3O4
- Copper(II) Oxide – CuO
- Copper(I) Oxide – Cu2O
- Tin(IV) Oxide – SnO2
- Titanium(IV) Oxide – TiO2
- Manganese(IV) Oxide – MnO2
Class Experiment
- Fill the bucket halfway with sand.
- Arrange the flower pot and ring stand so that the pot drapes over the middle of the bucket.
- Cover the opening in the pot with filter paper.
- Fill the container with roughly 1/2 inch of thermite powder.
- Thermite starting powder should be applied to the thermite.
- Put the Mg ribbon in the powder like a fuse and light it with the bunsen burner.
- Use tongs to lift red hot iron metal out of the sand.
Precautions
- The molten iron is really hot!! Take precautions!!
- To capture the molten thermite, never use water or glass in any combination.
- Make sure there is a dip in the sand for the molten metal to fall into.
- Ensure that the molten iron falls into the sand and does not touch the bucket’s sides.
- Do not exaggerate the emotion!!
- This is an extremely exothermic process! Everyone must take a step back!
- Keep any potentially combustible objects, such as papers, backpacks, and projector screens, at a safe distance.
Related Links
Flexural Strength- An Overview
Diffusion Coefficient| Mass Diffusivity
Dynamic Viscosity-An Overview
Electric Field Units & Definition
STEM Education| 5 Easy Key Points
Summary
To summarize everything in this article, the following are some important points:
- Thermite welding is the process of joining two or more workpieces by pouring molten metal between them.
- The process is also called the Goldschmidt process
- Aluminum metal is used as a reducing agent.
- When Aluminium is heated, it converts iron and chromium to a metallic state.
- This technique is used to weld railway rails, heavy machinery, and other comparable materials.
Frequently Asked Questions (FAQs)
Some of the frequently asked questions are given below
1. What is corrosion?
Corrosion is a natural phenomenon that occurs when chemically active metals are exposed to a wet environment. Corrosion is an oxidation reaction that occurs when water or moisture on the metal’s surface oxidizes with ambient oxygen.
2. What is anodizing?
Anodising is an electrolytic technique used to create thick oxide coatings on aluminum and related alloys. The oxide layer is generally 5 to 30m thick and is used to increase surface wear and corrosion resistance or as a decorative layer.
3. What is iron oxide?
Iron oxides are chemical compounds that are made up of iron and oxygen. They are found in hemoglobin and are utilized as iron ores, pigments, catalysts, and thermite. Iron oxides are low-cost and long-lasting pigments used in paints, varnishes, and colored concrete.
4. What is thermite welding?
Thermite welding is a type of welding that involves reducing metal oxides with aluminum powder and releasing a large quantity of heat. Thermit reaction is used to repair railway rails or broken machine components.
5. What is anodising?
Anodizing is an electrochemical technique that changes the surface of the metal into a beautiful, long-lasting, corrosion-resistant anodic oxide finish. Aluminum is suitable for anodizing, although other nonferrous metals such as magnesium and titanium can also be anodized.
6. Can work be negative?
Work can be negative if the applied force is directed in the opposite direction as the displacement of the moving item. To distinguish between positive and negative work, we must first grasp the relationship between applied force and work done.
7. What is the malleability of a material?
Malleability refers to a substance’s ability to distort under pressure. The material can be pounded or rolled into thin sheets if it is pliable. Metal malleability refers to the ability of a metal to deform under compression and take on a new shape.
8. flexural strength?
A material’s flexural strength is the highest bending force that can be applied to it before it yields. A transverse bending test employing a three-point flexural test methodology is the most popular method of assessing a material’s flexural strength.
9. gauge pressure formula
The pressure in a system that is more than atmospheric pressure is referred to as gauge pressure, also known as overpressure. Gauge pressure readings include atmospheric pressure since they are zero-referenced against ambient air (or atmospheric) pressure.
10. Instantaneous velocity?
The rate of change of position over a very short period of time is defined as instantaneous velocity.
11. Gravitational potential energy
The energy that an object has as a result of its location in a gravitational field is referred to as gravitational potential energy (w). If the object is pushed straight up at a constant speed, the force (F) necessary to hoist it to the height (h) is equal to the object’s weight (mg).
12. Define relativistic kinetic energy
According to the relativistic kinetic energy equation, when an object’s velocity approaches the speed of light, its energy approaches infinity. As a result, exceeding this speed restriction is impossible.
13. What are quarks in physics?
In physics, quarks are the fundamental building units of matter. They are most typically found inside protons and neutrons, the particles that form the nucleus of every atom in the universe. Based on present experimental findings, quarks appear to be really basic particles that cannot be further subdivided.
14. What is the ability to do work called?
Energy is the ability to do work, and work is the movement of anything against a force, such as gravity.
15. What is linear motion?
Linear motion is a one-dimensional motion along a straight line that can be represented mathematically with only one spatial dimension.
Author
Umair Javed
Umair has been working at Whatsinsight since 2020 as a content writer.
He has a Masters degree in Materials Science.
More Interesting Links
Is Titanium Magnetic?
Silicon Carbide – An Overview
Fluoroantimonic acid-Super Acid
Magnetic Permeability
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023