The ability of a substance to deform under pressure is known as malleability. If material is malleable, it can be hammered or rolled into thin sheets. Malleability in metals is the ability to deform under compression and take on a new shape. Metals are malleable because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond. Gold and silver are both extremely malleable metals. Nickel, on the other hand, is the least malleable metal.
Table of Contents
What makes Metals Malleable?
Metals are malleable due to the properties of their crystalline structure in the solid-state. Most metals have similar properties, which are derived from the general valency of the atoms. Metals are malleable because of the metallic bonds that hold the atoms together. Metallic bonds, which are characterized by a “sea” of electrons that easily move from one atom to the next, allow metal atoms to slide past each other when a force is applied. The force can be generated by a hammer blow, the impact of a fall, high pressure from being squeezed, or a collision. Some examples of malleable materials are listed below:
Elements with close-packed crystal structures [hexagonal close-packed (hcp) or face-centered cubic (fcc)] are more malleable in general than those with more open structures, such as body-centered cubic (bcc) (bcc). Gold, silver, and magnesium, for example, are more malleable than vanadium or chromium.
Atoms in densely packed structures are arranged like stacked flat sheets, allowing planes to slip past each other when a force is applied. Body-centered structures, on the other hand, are more like corrugated sheets that resist slipping.
Degree of Malleability
The degree of malleability varies greatly between metals and metal mixtures, also known as alloys.
Multiple factors can influence the malleability of a metal or alloy, but the strength of the metallic bond and temperature are two of the most important.
In addition, Harder metals have a lower malleability than softer metals. Pure gold and silver, for example, are extremely soft and malleable. Hard metals like tungsten, iridium, osmium, and chromium are not easily malleable. Because of their crystal structures, metals are hard and non-moldable. Because the atom rows in the crystals do not line up, there are many grain boundaries. Under pressure, there is no easy way for atoms to slide past one another.
Summary
- A malleable material is one that can be easily formed into a thin sheet by hammering.
- The most malleable metal is gold.
- Malleable metal has the ability to deform under compressive stress.
- Brittle material is one that fractures after only a small amount of plastic deformation.
- Ductile material is a material that can easily be stretched into a wire when pulled.
Frequently Asked Questions
1. How does solar power work?
Solar energy is transformed into power by converting the sun’s energy. The sunlight helps us to produce two forms of energy for human use: electricity and heat. Both are made possible by the usage of solar panels, which range in size from residential rooftops to acre-long “solar farms” on rural terrain.
2. What is liquid pressurized gas?
Liquefied petroleum gas (LPG), like coal, natural gas, and crude oil, is a fossil fuel. Unlike the others, LPG is never found on its own in nature; it is always found in combination with either natural gas or crude oil. In reality, LPG is a byproduct of the processing of these fuels. Approximately 60% of LPG is produced during natural gas extraction, with the remaining 40% recovered during crude oil refining.
3. What is energy density in physics?
The quantity of energy stored in a specific system or region of space per unit volume is referred to as energy density in physics.
4. How can we increase the efficiency of the Otto cycle?
The efficiency of an Otto engine can be improved by running it on lean fuel (which consumes additional air) or by increasing the compression ratio.
5. Why is thermal mass important for passive solar?
Thermal mass is an important and complementary component of passive solar design. Thermal mass refers to a material’s ability to receive, store, and release heat energy from the sun.
6. Do aerosols block sunlight?
Aerosols are microscopic particles that float in the Earth’s atmosphere. They have a cooling impact because they obstruct sunlight that would otherwise reach the globe.
7. How is static electricity useful for solar panels?
Researchers at MIT have invented a novel approach that utilizes static electricity to clear dust from solar panels, potentially saving 45 billion liters of water every year. Check the full article.
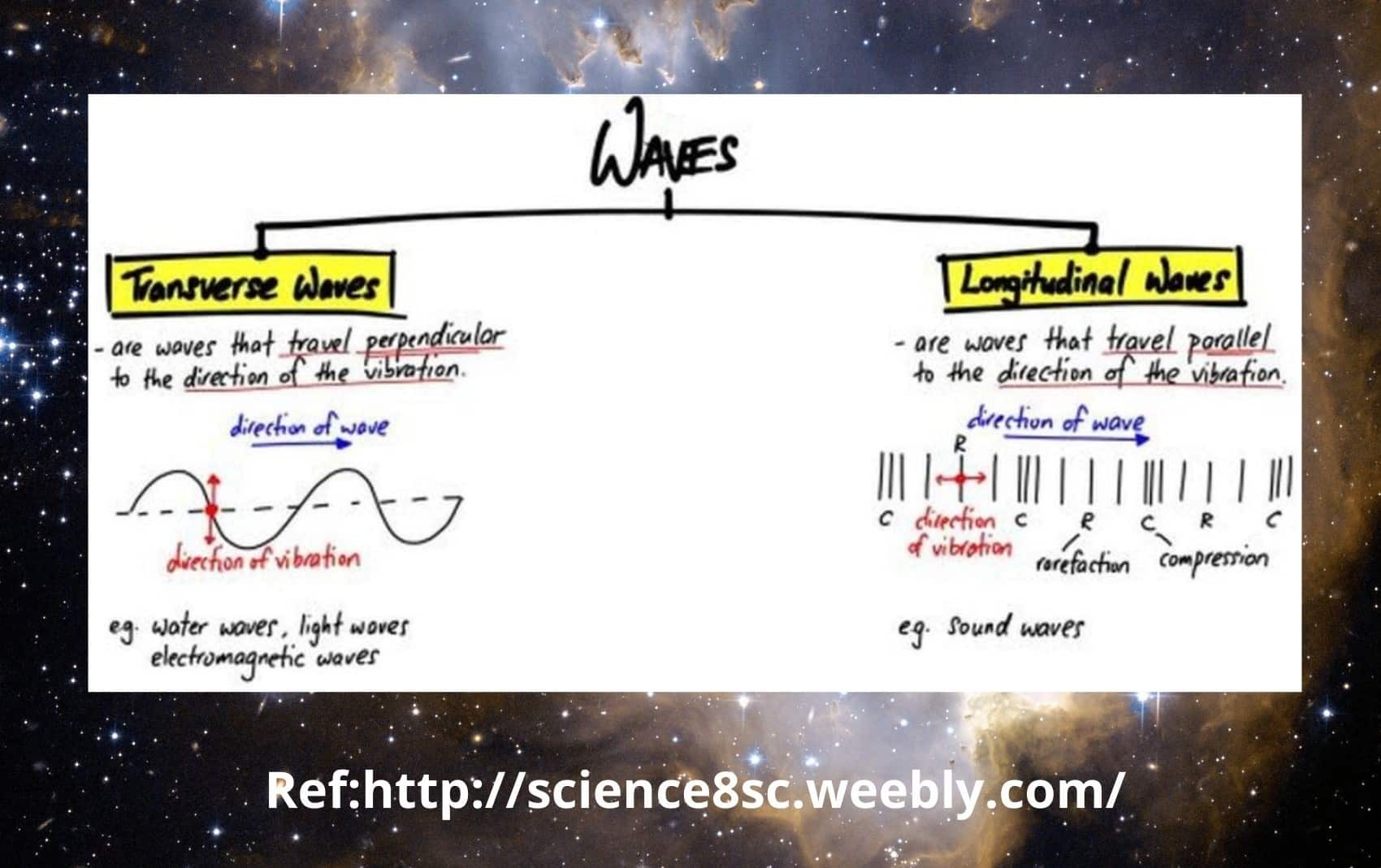
8. Crest of a wave?
The crest of a wave is the section of the transverse waves above the mean level. The trough of a transverse wave is the segment of the wave below the mean level. The wavelength is defined as the distance between two consecutive crests or troughs.
9. Centripetal acceleration?
Centripetal acceleration is the acceleration experienced by a moving body traveling in a circular route. It is pointing towards the circle’s center.
10. Damped oscillation?
A damped oscillation is one that fades away over time. Examples include a swinging pendulum, a weight on a spring, and a resistor-inductor-capacitor (RLC) circuit.
More Links
Is Titanium Magnetic?
How many valence electrons does iron have?
Zinc Metal| Properties and Uses
Is Chlorine a metal?
Is Aluminum Conductive?
Flexural Strength- An Overview
The Spring Constant – Hooke’s Law
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023