Barium sulfate has the chemical formula BaSO4 and is an inorganic substance. Barium sulfate is used as an X-ray contrast medium and is added to concrete to improve radiation resistance. In addition, It is the primary source of barium and is widely utilized in the chemical and medicinal industries since it is the only nontoxic salt of barite.
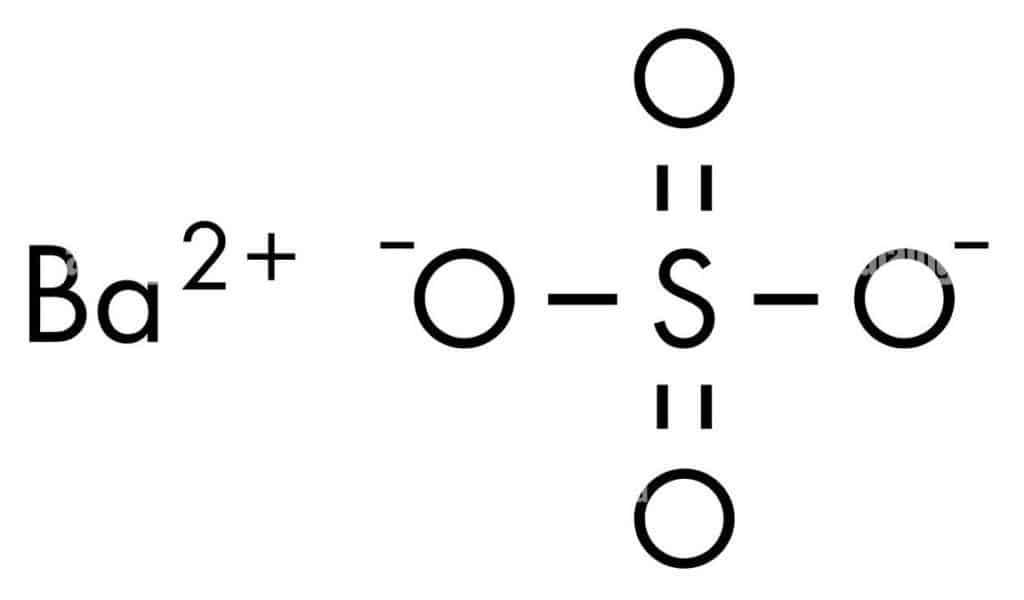
Some minerals contain barium sulfate, notably barite. The purification and extraction of barium sulfate from the mineral is accomplished by a technique known as carbothermal reduction, which involves heating the material with coke and then treating the result of these reactions with sulfuric acid.
| Technical Name of compound | Barium sulfate (BaSO4 ) |
| Appearance | odorless, white or yellow crystalline solid or powder(at room temperature) |
| Melting point | 1580 ºC |
| Structure | orthorhombic lattice |
| Density | 4.25 g mL-1 |
| Uses | Barium sulfate is used in medicine, as a cocktail in the radio-opaque diagnosis. |
Table of Contents
Element Barium
The periodic table classifies barium as an alkaline-earth metal because it is soft, ductile, and silvery-white. Barium compounds are employed in a range of settings and industries and have been studied since the 1600s. It is a relatively “heavy” element, which has ramifications for its applications and compounds.
Related Links
Molar Mass of Acetic Acid| Easy-Explanation
Acetyl Group| 9 Key Points-Easy Explanation
Hydrogen Bond| Definition & Easy Explanation
Is HCN Polar or Nonpolar| Simple Explanation
Liquid Oxygen-Cryogenic Liquid
Disclaimer
Whatsinsight.org‘s blog and everything published on it are provided solely as an information resource. The blog, its authors, and affiliates accept no responsibility for any accident, injury, or damage caused by following the information on this website, in part or in whole. We do not recommend using any chemical without first consulting the manufacturer’s Material Safety Data Sheet and following the safety advice and precautions on the product label.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



