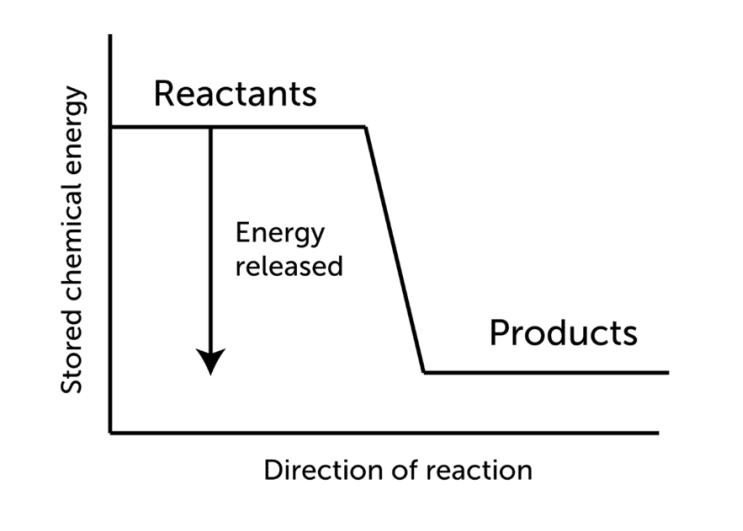
The principle of Le Chatelier is an observation regarding reaction chemical equilibria. The principle states that when a change in concentration, pressure, or temperature is applied to a system that is at equilibrium, the equilibrium position moves in a direction that seeks to decrease the influence of the change.
Le Chatelier’s Principle Simple Example
When the equilibrium of a chemical system is disrupted by stress, the system responds to release the stress. Changes in the concentration of reactants or products, changes in the system’s temperature, or changes in the system’s pressure are all examples of stresses on a chemical system.
In every situation, the change in the equilibrium position favours either the forward or backward response. When the forward reaction is favoured, product concentrations grow while reactant concentrations drop. When the reverse reaction is favoured, product concentrations fall while reactant concentrations increase.
Le Chatelier’s Principle Examples
Here are some examples of Le Chatelier’s Principle:
- Acid-base equilibrium: In an acid-base equilibrium, increasing the concentration of either the acid or the base will shift the equilibrium in the opposite direction to counteract the change. For example, if hydrochloric acid (HCl) is added to a solution of sodium hydroxide (NaOH), the reaction will shift to favour the production of water and sodium chloride (NaCl) to consume the excess HCl.
- Haber process: The Haber process is used to produce ammonia from nitrogen and hydrogen gases. According to Le Chatelier’s Principle, increasing the pressure of the reaction vessel will shift the equilibrium to favour the formation of ammonia, as the forward reaction involves a decrease in volume. Similarly, increasing the temperature will shift the equilibrium to favour the endothermic reverse reaction.
- Solubility of gases: The solubility of gases in liquids is also subject to Le Chatelier’s Principle. When the pressure of a gas above a liquid is increased, the equilibrium will shift to favour the dissolving of the gas into the liquid to counteract the increase in pressure. For example, carbonated beverages contain dissolved carbon dioxide (CO2) gas that is held in solution under pressure.
- Equilibrium reactions: Many reactions that are at equilibrium are subject to Le Chatelier’s Principle. For example, the reaction between nitrogen dioxide (NO2) and dinitrogen tetroxide (N2O4) is at equilibrium. If the concentration of NO2 is increased, the reaction will shift to favour the reverse reaction to consuming the excess NO2.
- Buffer systems: Buffer systems are used to maintain a constant pH in biological and chemical systems. According to Le Chatelier’s Principle, if the pH is disturbed, the buffer system will respond by releasing or absorbing H+ ions to counteract the change and restore equilibrium. For example, the bicarbonate buffer system in the blood helps to maintain a constant pH despite changes in acid or base concentration.
Real-Life Examples of Le Chatelier’s Principle
Le Chatelier’s Principle is a fundamental principle in chemistry that describes how a system at equilibrium will respond to changes in conditions. Here are some real-life examples of Le Chatelier’s Principle:
- Carbon monoxide production: In the Haber process, which is used to produce ammonia, carbon monoxide is produced as a byproduct. The production of carbon monoxide is an exothermic reaction, meaning it releases heat. According to Le Chatelier’s Principle, increasing the temperature will shift the equilibrium to favour the reactants, which will decrease the amount of carbon monoxide produced.
- Blood pH regulation: The human body maintains a pH of around 7.4 in the blood, which is critical for proper physiological function. One of the ways the body regulates pH is through the use of the bicarbonate buffer system. According to Le Chatelier’s Principle, if the pH of the blood starts to decrease (become more acidic), the body will produce more bicarbonate ions (HCO3-) to shift the equilibrium back towards the alkaline side.
- Solubility of gases: According to Henry’s law, the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the liquid. According to Le Chatelier’s Principle, if the partial pressure of a gas is increased, the equilibrium will shift to favour the dissolving of the gas into the liquid, increasing its solubility.
- Chemical reactions in the human body: Many chemical reactions in the human body are subject to Le Chatelier’s Principle. For example, the reaction between carbon dioxide and water to form bicarbonate ions is an important reaction that helps regulate pH in the blood.
- Equilibrium in reversible reactions: Reversible reactions, such as the Haber process and the formation of esters in organic chemistry, are subject to Le Chatelier’s Principle. Changes in temperature, pressure, or concentration can shift the equilibrium to favour either the reactants or the products, altering the yield of the reaction.
Frequently Asked Questions
- What is Le Chatelier’s Principle?
Answer: Le Chatelier’s Principle is a fundamental principle in chemistry that describes how a system at equilibrium will respond to changes in conditions. - What is the effect of increasing the concentration of a reactant on a system at equilibrium, according to Le Chatelier’s Principle?
Answer: Increasing the concentration of a reactant will shift the equilibrium to favour the formation of products. - How does Le Chatelier’s Principle explain the effect of increasing temperature on the solubility of gases in liquids?
Answer: According to Le Chatelier’s Principle, increasing the temperature will shift the equilibrium to favor the dissolving of the gas into the liquid, increasing its solubility. - In the Haber process, what is the effect of increasing pressure on the yield of ammonia?
Answer: According to Le Chatelier’s Principle, increasing the pressure will shift the equilibrium to favor the formation of ammonia, increasing the yield. - How does Le Chatelier’s Principle explain the effect of adding a catalyst to a reaction system at equilibrium? Answer: Le Chatelier’s Principle states that a change in conditions will cause the system to shift in a direction that opposes the change. Since adding a catalyst does not change the equilibrium constant of the reaction, the equilibrium position will not be affected. However, the catalyst can increase the rate of the forward and backward reactions, which will allow the system to reach equilibrium more quickly.
Related Questions
1. What is a chemical reaction?
A chemical reaction is a process that results in the chemical change of one set of chemical substances into another set of chemical substances.
Reactants or reagents are chemicals (or substances) that are initially involved in a chemical reaction. Chemical reactions generally involve a chemical change and the formation of one or more products with characteristics distinct from the reactants.
2. What is atmospheric pressure?
The force exerted by the weight of the atmosphere per unit area is known as atmospheric pressure. One atmosphere equals 1,013 millibars of mercury, or 760 millimeters (29.92 inches). As height rises, atmospheric pressure decreases.
3. Room temperature definition in simple words?
The range of temperatures in which humans are comfortable is referred to as room temperature. A minimum room temperature of 20 °C (68 °F) is recommended for babies, children, the elderly, and those who are unwell, according to the World Health Organization (WHO). It has no set temperature, however, it is usually between 20 and 25 degrees Celsius (68 and 77 degrees Fahrenheit).
4. Enthalpy in simple words?
Enthalpy is a thermodynamic quantity that measures a system’s total heat content. It is the total of the system’s internal energy, as well as the product of pressure and volume.
The product of the system’s pressure, P, and volume, V, plus the internal energy, E, is the enthalpy, H.
H = E + PV is the mathematical formula for it.
5. Explain Chemical equilibrium?
Chemical equilibrium is a state in which no net change in the quantities of reactants and products occurs during a reversible chemical process.
There is no net change in the quantities of chemicals involved when the two opposing reactions occur at identical rates or velocities. At this point, the reaction can be regarded as complete; that is, the maximal conversion of reactants to products has been achieved for a given reaction state.
6. What is the concentration gradient definition in biology?
The progressive shift in the concentration of solutes is referred to as a concentration gradient.
The steeper the concentration gradient and the faster the molecules of a substance disperse, the greater the difference. Diffusion comes to an end when the concentration of the chemical in both regions is the same.
7. Definition of osmosis?
Osmosis is a process in which solvent molecules flow from a less concentrated solution to a more concentrated solution across a semipermeable membrane.
8. What is Hess’s law equation?
The heat absorbed or evolved (or the change in enthalpy) in every chemical reaction is a constant quantity that is independent of the reaction pathway or the number of steps required to complete the reaction, according to Hess’s law of heat summation.
More Links
How Much is a Liter of Water?
Thermal Energy Equation- Simple Overview
Laminar Flow| Physics
Conduction in Physics| Easy Examples
Combustion Reactions| Introduction, Reaction, & Facts
Osmosis Definition
Hydrogen Molar Mass
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023