A heat engine is a device that extracts heat from a source and converts it into mechanical work that may be utilized for a variety of purposes. A steam engine on an old-style railway, for example, can generate the energy required to drive the train. Power plants, internal combustion engines, steam engines, the diesel engine, and the gasoline (petrol) engines in an automobile. are examples of heat engines.
A heat engine is made up of a heat source (high-temperature reservoir or hot reservoir) and a heat sink (low-temperature reservoir or cold reservoir), as shown schematically in Figure 1. (Figure).
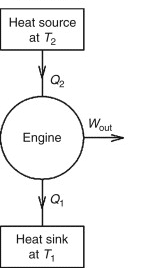
The heat engine works on the simple concept of absorbing heat from a heat source (hot reservoir) of Kelvin temperature, using part of that energy to generate useful work W, and then discarding the remaining energy as heat into a heat sink (cold reservoir) of Kelvin temperature.
Table of Contents
What is Heat Engine?
An engine is a machine that transforms the stored energy in fuel into force and motion. Engines produce heat by burning fuels; they are frequently referred to as heat engines. The energy contained in an engine can be used to power manufacturing machinery, vehicles, boats, or locomotives. The combustion process comprises a chemical reaction in which the fuel burns oxygen in the air to produce carbon dioxide and steam. (In general, engines contribute to air pollution since fuel isn’t always 100 percent pure and doesn’t burn exactly cleanly.)
There are two main types of heat engines: external combustion and internal combustion:
- External combustion engine
- Internal combustion engine
In an external combustion engine, the fuel burns outside of the engine, away from the primary part that generates force and motion. On the other hand, the fuel in an internal combustion engine burns within the cylinder. An example of an external combustion engine is a steam engine, while an example of an internal combustion engine is a car engine.
Heat Engine Efficiency
The efficiency (actual or theoretical) of an engine is determined by the maximum and minimum temperatures at which it works. The efficiency of a Carnot engine running between Tmax (its highest temperature) and Tmin (its minimum temperature) is calculated as follows:
The efficiency of heat engine = (Tmax – Tmin)/Tmax (Both temperatures are measured in kelvin (K)
The Carnot cycle
A Carnot cycle is a closed thermodynamic cycle that is ideal and reversible. Isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression are all performed sequentially. A heat engine that works according to the Carnot cycle is known as a Carnot engine. A Carnot engine is a heat engine that operates on the Carnot cycle.
In addition, The Carnot cycle is a theoretical engine cycle that has the best efficiency of any heat engine. It defines the maximum efficiency of a heat engine, and all practical engines will be less efficient than a Carnot engine.
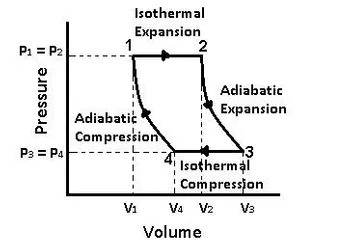
Isothermal Expansion
The temperature remains constant throughout the isothermal expansion process, but volume increases and pressure lowers. Heat enters the system during this period. This heat might come from the combustion of fuel, as in an internal combustion engine, or it can come from somewhere else. The gas inside the system expands to maintain the temperature constant.
Adiabatic Expansion
During this stage, the gas continues to expand quickly with no further heat input. As it expands, it has an effect on the environment, lowering the internal energy of the system and lowering the pressure and temperature.
Isothermal Compression
The temperature remains constant during this stage, but volume falls and pressure rises. Temperature is kept constant by keeping the system in contact with a cool reservoir, allowing heat to escape as effort is done to compress the gas.
Adiabatic Compression
The cold reservoir is removed in this stage, and the volume continues to drop while the pressure and temperature rise.
What is Heat Pump?
Heat pumps operate by pumping or moving heat from one location to another utilizing a compressor and a circulating structure of liquid or gas refrigerant, which extracts heat from outside sources and pushes it within. Heat pumps provide several benefits to your house.
Summary
- Engines that produce heat by burning fuels are frequently referred to as heat engines.
- The work done by a heat engine is the difference between the heat taken from the hot reservoir and the heat released into the cold reservoir.
- The heat engine’s efficiency is determined by the ratio of work done by the engine to heat absorbed from the hot reservoir.
Related Topics
First Law of Thermodynamics
Flaring of Gases| Why We Need It?
Conduction in Physics
is burning wood a chemical change?
What is the Specific Heat of Air?
Specific Heat Formula & Concept
Frequently Asked Questions
1. What is the Carnot cycle?
The Carnot Cycle depicts the most efficient heat engine feasible, comprising two isothermal and two adiabatic operations. It is the most efficient heat engine feasible under the constraints of physics.
2. What is the Second law of thermodynamics?
According to the second law of thermodynamics, hot objects always cool until anything is done to stop them. It illustrates a fundamental and obvious fact about the world that a system’s entropy (disorderliness) continually increases.
3. Is Liquefied Petroleum Gas the same as natural gas?
Liquefied Petroleum Gas (LPG) is not natural gas since it contains propane, whereas natural gas contains methane. LPG is produced as a byproduct of the natural gas and crude oil refining operations. Once processed, LPG is stored as a liquid under pressure in gas bottles or tanks.
4. What is liquified natural gas?
Liquefied natural gas (LNG) is natural gas that has been liquefied for transportation and storage.
Natural gas is nearly completely composed of methane, with tiny quantities of other gases such as ethane, propane, butane, and pentane. A molecule of methane has one carbon atom and four hydrogen atoms.
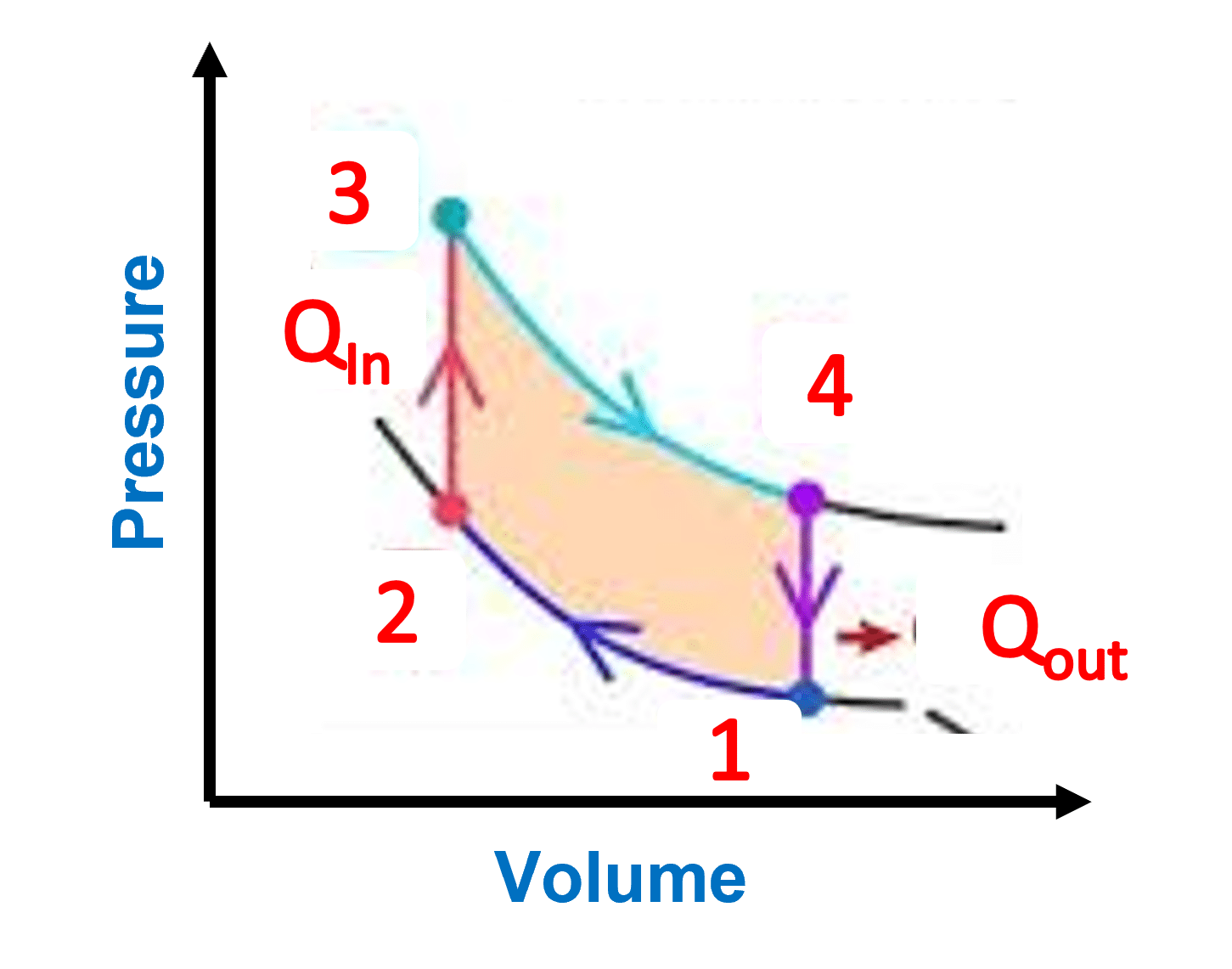
5. What is a pressure-volume diagram?
The pressure-volume diagram (abbreviated as the PV diagram) is a graphical representation of pressure fluctuations in a closed system with regard to volume. P-V diagrams may be used to calculate the efficiency of a system as well as the work done by or on the system.
6. Thermal mass?
A substance’s thermal mass is its ability to absorb, store, and release heat. Different thermal mass materials absorb varying amounts of heat and absorb and re-radiate it for varying lengths of time. Because a brick wall has a higher thermal mass than a timber-framed hollow wall, it absorbs more heat than a timber-framed wall of the same thickness.
7. Hydrog turbines?
Hydro turbines are equipment used in hydroelectric power plants to transmit energy from flowing water to a rotating shaft, where it is converted into electricity. In response to the infusion of water into their blades, these turbines revolve or spin.
8. What is the Otto cycle?
The Otto cycle is a type of engine cycle that needs four piston strokes: induction, compression, ignition, and exhaust. The fuel and air mixture is compressed before combustion is induced by an electrical spark or other means.
9. Carbon footprint?
A carbon footprint, often known as a CO2 footprint, is the total amount of greenhouse gas emissions (including carbon dioxide and methane) produced by an individual’s actions during a given time period, typically a year.
10. What is an alternative fuel vehicle?
An alternative fuel vehicle (AFV) is a vehicle that runs on fuels other than gasoline and diesel. Alternative fuels are derived from non-petroleum sources. The fundamental purpose of using alternative fuels is to lessen our dependency on imported oil; hence, alternative fuels are produced from renewable sources.
More Interesting Topics
Solar Thermal Power Plant| Introduction and Working
Greenhouse Effect| Definition And 5 Key Factors
Light Energy| 5- Easy Examples
Convection| Atmospheric Motions in the Vertical Direction
Hess’s Law: Statement, Equation, & Examples
What Is Auxiliary Heat| 5 Easy Key Points
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023