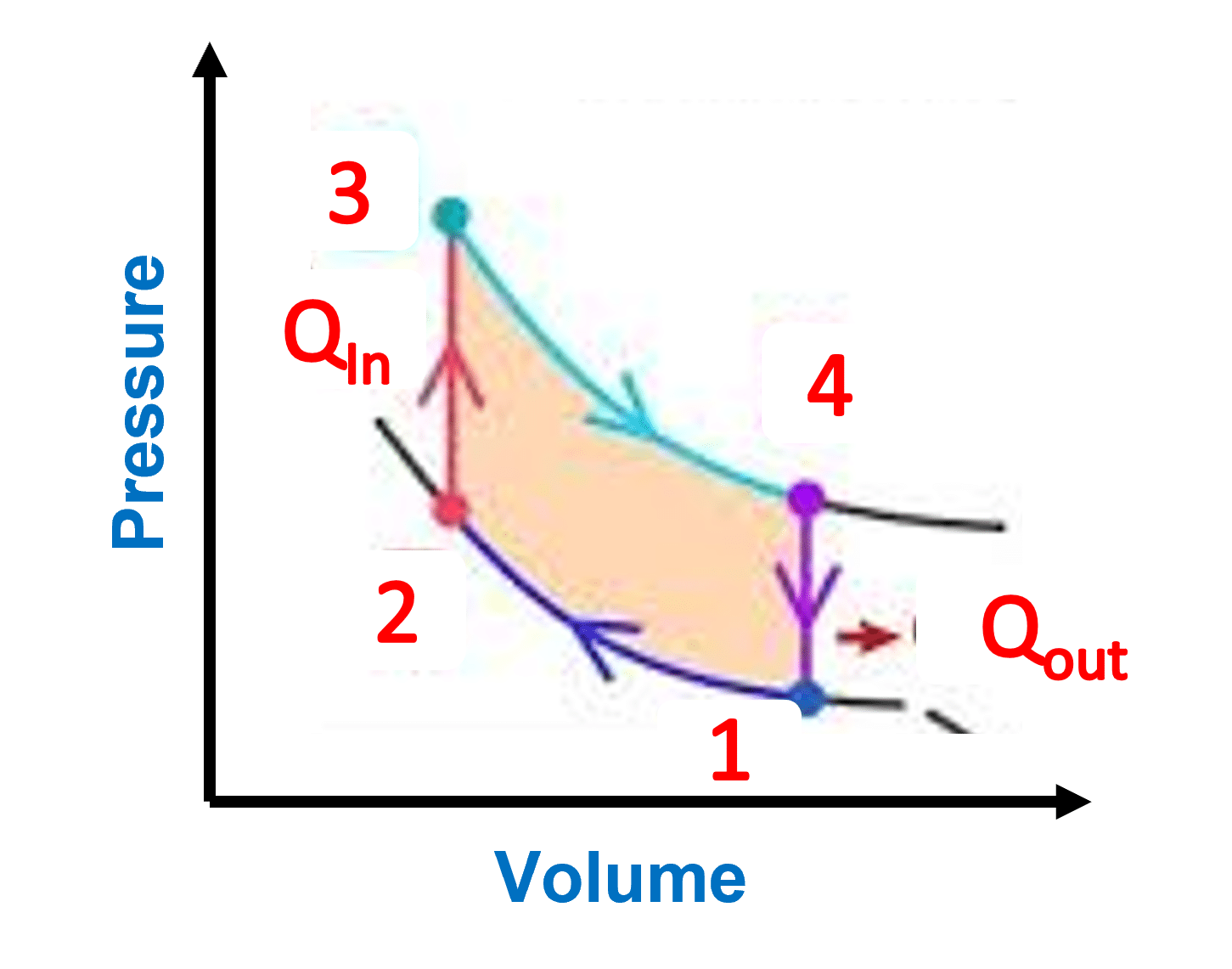
The adiabatic process is a thermodynamic process in which no heat is exchanged from the system to its surroundings during expansion or compression. Often, adiabatic processes are carried out in an insulated container, where the process is too fast for heat to be transported.

A heat engine that compresses a gas enclosed within a cylinder is an example of adiabatic heating. The temperature rises as a result of gas compression.
In addition, A simple example of an adiabatic system is Icebox which prevents heat to enter or exit from the system.
Table of Contents
Daily Life Examples of Adiabatic Process
| Example | Explanation |
|---|---|
| Compression and expansion of gases | When you pump up a bicycle tire, you compress the air inside. Similarly, when you use a spray can, the contents expand rapidly due to an adiabatic process. |
| Atmospheric cooling | When air rises in the atmosphere, it expands and cools adiabatically. This is why the tops of mountains are colder than their bases, even if the sun is shining. |
| Diesel engines | Diesel engines work by compressing air in a cylinder until it gets hot enough to ignite the fuel. This is an example of an adiabatic process because the compression is done quickly enough that there is no time for heat to escape. |
| Air conditioning and refrigeration | These systems work by compressing and expanding gases. When a gas is compressed, it heats up adiabatically. When it is allowed to expand, it cools down adiabatically. |

What is Adiabatic Process?
An adiabatic process is a sort of thermodynamic process in which no heat or mass may leave or enter the system.
Energy is transferred from an adiabatic system in the form of work done. The adiabatic walls of the system prevent heat transfer. The working fluid within the system can perform work by causing the system’s walls to move to and fro or up and down. The following are the necessary conditions for the adiabatic process to occur:
- The system must be completely isolated from its surroundings.
- The process must be completed quickly so that there is no time for a significant heat transfer to take place.
The first law of thermodynamics can be expressed mathematically as:
Change in the system’s internal energy (ΔU)= Heat (Q) + work done on the system (W)
Since the heat in the adiabatic process is zero
ΔU= W
Isotherms and adiabats curves may appear similar at first sight, however as shown in Figure 1, adiabats have steeper slopes As a result, the ideal gas law alters significantly, as seen in the equation below:
PVγ=constant
p represents pressure, V represents volume, and (gamma) represents specific heat ratio, which is 1.67 for a monatomic gas and 1.40 for a diatomic gas.
Important Definitions
- Adiabatic cooling: When the pressure in an adiabatically isolated system is reduced, the gas expands and does work on its surroundings. As a result, the temperature drops.
- Adiabatic heating: When work is done on an adiabatically isolated system, the pressure rises, and hence the temperature rises.
Joke of the Day 🙂
Why did the thermodynamics textbook feel cold?
Because it was adiabatic and couldn’t exchange heat with its surroundings!
Exams Related Questions
| 1. | What is an adiabatic process? | An adiabatic process is a thermodynamic process in which there is no heat transfer between the system and its surroundings. |
| 2. | What is the first law of thermodynamics? | The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or converted from one form to another. |
| 3. | What is the relationship between work and heat in an adiabatic process? | In an adiabatic process, all the work done on or by the system goes into changing the internal energy of the system and there is no heat transfer. |
| 4. | What is the equation for the first law of thermodynamics for an adiabatic process? | The equation for the first law of thermodynamics for an adiabatic process is ΔU = -W, where ΔU is the change in internal energy of the system and W is the work done on or by the system. |
| 5. | What is the difference between an adiabatic process and an isothermal process? | In an adiabatic process, there is no heat transfer between the system and its surroundings, whereas in an isothermal process, the temperature of the system remains constant throughout the process. |
| 6. | What is the relationship between the pressure and volume of a gas in an adiabatic process? | In an adiabatic process, the pressure and volume of a gas are related by the equation PV^γ = constant, where γ is the ratio of specific heats of the gas. |
| 7. | What is the value of γ for an ideal gas? | The value of γ for an ideal gas is 1.4. |
| 8. | What is the significance of the adiabatic exponent in an adiabatic process? | The adiabatic exponent determines how much the pressure and volume of a gas change for a given change in temperature in an adiabatic process. |
| 9. | What are some examples of adiabatic processes in everyday life? | Some examples of adiabatic processes in everyday life include the compression or expansion of a gas in a cylinder, the free expansion of a gas into a vacuum, and the adiabatic cooling of the atmosphere during expansion. |
| 10. | What are some practical applications of adiabatic processes? | Some practical applications of adiabatic processes include air conditioning and refrigeration systems, gas turbines, and diesel engines. |
| 11. | What is the difference between an adiabatic process and an adiabatic reversible process? | An adiabatic reversible process is a special case of an adiabatic process in which the process is also reversible, meaning that the system can be returned to its initial state by a reversible process. |
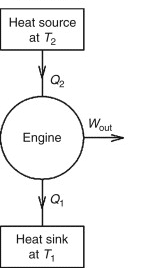
| 12. | What is the Carnot cycle, and how is it related to adiabatic processes? | The Carnot cycle is a theoretical cycle that consists of four reversible processes, two of which are adiabatic. It is a model for the most efficient heat engine possible, and it demonstrates the importance of adiabatic processes in thermodynamics. |
| 13. | How does an adiabatic process differ from an isentropic process? | An isentropic process is a reversible adiabatic process, meaning that it is |
Frequently Asked Questions
1. What is an adiabatic process?
An adiabatic process is one that does not involve any heat transfer. This does not imply a constant temperature, but rather that no heat is moved into or out of the system.
2. Which quantity remains constant in an adiabatic process?
In an adiabatic process, the total heat of the system remains constant.
3 What is the difference between the isothermal and adiabatic processes?
An isothermal process is one in which the temperature of the system remains constant. The entire procedure is carried out at a steady temperature. The adiabatic process, on the other hand, is a thermodynamic process in which no heat transfer occurs between the system and its surroundings, implying that there is no exchange of heat across the system’s walls.
4. PV diagram?
The pressure-volume diagram (abbreviated as the PV diagram) is a graphical representation of pressure fluctuations in a closed system. P-V diagrams may be used to calculate the efficiency of a system as well as the work done by or on the system.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023