Nitrogen (N) is a colourless, odourless, relatively unreactive gaseous element that makes up 78% of the air by volume, occurs in many compounds, and is a necessary component of proteins and nucleic acids: used in the production of ammonia and other chemicals, as well as as a refrigerant. A nitrogen atom, according to the Bohr model, has a central nucleus made up of seven protons and seven neutrons, surrounded by seven electrons.
The first two electrons in the electron configuration for nitrogen will be in the 1s orbital. Since the 1s orbital can only hold two electrons, the next two electrons for Nitrogen enter the 2s orbital. The remaining three electrons will fill the 2p orbital. As a result, the Nitrogen electron configuration will be 1s2 2s2 2p3, as shown in the diagram below.
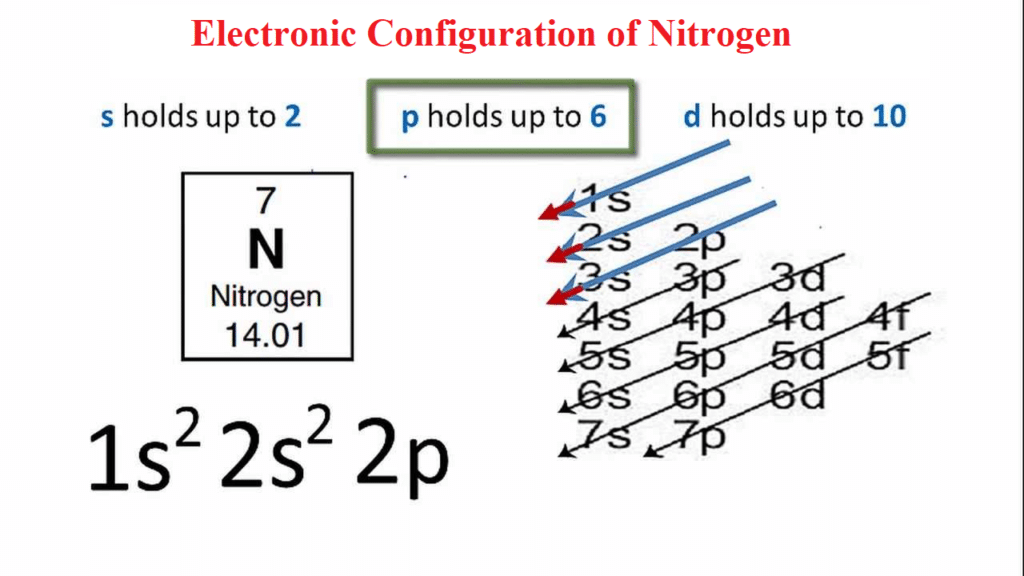
Table of Contents
Nitrogen- Key Points
Nitrogen isotopes: Nitrogen-14 and nitrogen-15 are the two stable isotopes
| Name of element | Nitrogen (N) |
| Atomic number | 7 |
| Atomic mass | 14.0067 (amu, g/mol) |
| Nitrogen electron configuration | 1s2 2s2 2p3 |
| Density | 1.25*10-3 g.cm-3 at 20°C |
| Melting point: | -210 °C |
| Boiling point: | -195.8 °C |
Importance of Nitrogen for life
The nitrogen cycle is essential because nitrogen is a necessary nutrient for life on Earth. Furthermore, nitrogen is a necessary component of amino acids, the building blocks of proteins, and nucleic acids, the building blocks of genetic material (RNA and DNA).
Is Nitrogen a non-metal?
Nitrogen is a nonmetal and the lightest member of Periodic Table Group 15, also known as the pnictogens.
Nitrogen Isotopes
Nitrogen is composed of two stable isotopes, 14N and 15N. (atomic masses of 14 and 15, respectively). 14N is the more abundant of the two, accounting for 99.63% of all nitrogen in nature. Physical, chemical, and biological processes can tell the difference between the two isotopes.
Summary
- Nitrogen has a central nucleus made up of seven protons and seven neutrons, surrounded by seven electrons.
- The electron configuration of Nitrogen is 1s2 2s2 2p3
Related Topics
Neon Element| Properties & Uses
Sulfur trioxide (SO3)
Electron Configuration for Calcium
Titanium Electron Configuration
Recommended Video
Frequently Asked Questions
1. What is the sulfur electronic configuration?
Sulfur electronic configuration is 1s2 2s2 2p6 3s2 3p4
2. What is calcium’s electronic configuration?
Calcium electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2
3. How many electrons does oxygen have?
A single oxygen atom has eight protons, eight electrons, and eight neutrons.
Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons. Its mass is 15.99491461956 u.
4. How many electrons does helium have?
A single helium atom has two protons, two electrons, and two neutrons. The second element in the periodic table is helium. Because s subshells may store up to two electrons, both electrons fit into the 1s subshell, resulting in the electron configuration for helium atoms being 1s2.
More Interesting Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023




