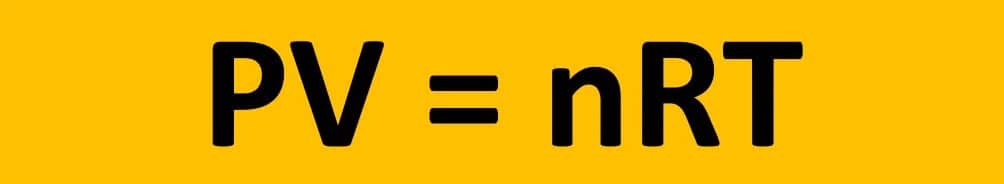Oxygen (O) is a nonmetallic element in Periodic Group 16 (VIa). It is a colourless, odourless, and tasteless gas. Oxygen is classified as a gas and nonmetal. It has a density of 1.429g/L and an atomic weight of 15.999. At room temperature and pressure, oxygen is made up of two oxygen atoms that combine to form dioxygen (O2), a colourless, tasteless, and odourless gas. Most lifeforms on Earth, including humans, require oxygen to survive. It is required for respiration in both animals and plants. It can be found in the air we breathe as well as the water we drink.
The atomic number of oxygen is 8, which means that an oxygen atom has eight protons in its nucleus. When writing the electron configuration for oxygen, the first two electrons will be in the 1s orbital. Since the 1s orbital can only hold two electrons, the next two electrons are placed in the 2s orbital. The remaining four electrons will enter the 2p orbital. As a result, the electronic configuration for oxygen will be 1s2 2s2 2p4.
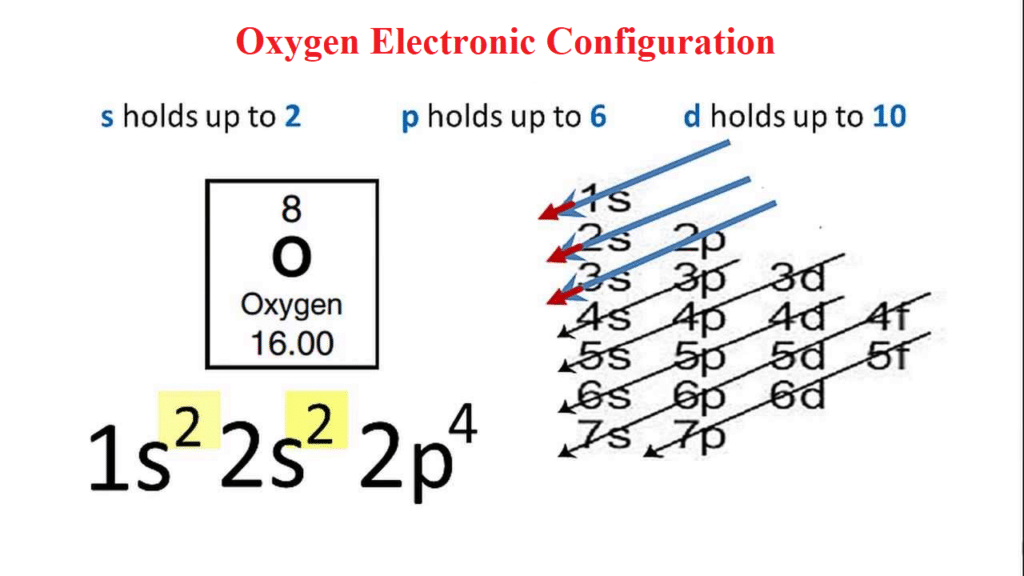
Table of Contents
Oxygen-Key Points
- Atomic number: 8
- Atomic weight: 16 amu
- Density: 1.429 g/L
- Oxygen isotopes are 18O, 17O, and 16O.
- State at 20°C: gas
- Group: 16
- Period: 2
Element Oxygen
Oxygen exists in nature as a molecule. To form dioxygen or O2, two oxygen atoms form a strong covalent double bond. Normally, oxygen is found as a molecule. It is known as dioxygen.
Summary
- The electron configuration of oxygen is 1s2 2s2 2p4.
- Oxygen is a nonmetal that belongs to one of the periodic table’s 16 element groups. Oxygen is a tasteless, colourless, and odourless gas that is vital to animals, humans, and all living organisms on Earth.
- Oxygen isotopes are 18O, 17O, and 16O.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023