Benzene hexachloride (BHC), also known as lindane or hexachloride, is an organic molecule with the chemical formula C6H6Cl6 which is an isomer of hexachlorocyclohexane. It is a colorless substance that has a somewhat musty odor. This is another organochlorine chemical with several applications in agriculture and the pharmaceutical sector.
| Molecular formula | Benzene hexachloride (C6H6Cl6) |
| Molecular weight | 290.814 grams per mole |
| Density | 1.89 |
| The melting point of benzene hexachloride | 113 degrees Celsius |
| Boiling point | 323 degrees Celsius. |
As benzene compounds do not normally perform addition reactions quickly, the process necessary to make benzene hexachloride requires an additional outside stimulant, which is light. And thus, the addition of chlorine to benzene produces various isomers, including alpha, beta, and gamma isomers. The isomer that is helpful as an insecticide and has a wide range of applications is known as lindane, whereas the gamma isomer of benzene hexachloride is known as gammaxene.
Table of Contents
Benzene hexachloride Molecular Geometry
Benzene hexachloride contains six carbon atoms, six hydrogen atoms, and six chlorine atoms, indicating that the benzene double bonds have been substituted with chlorine compounds.
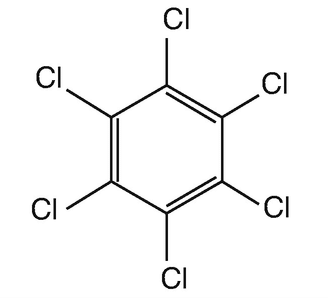
What is Benzene?
Benzene, having the chemical formula C6H6, has a molar mass of little more than 78 grams/mole. Benzene prefers to exist in a liquid form at ambient temperature. It is colorless and has a strong gasoline-like odor. Benzene is also a very flammable substance. This carcinogenic chemical is often used as an additive in gasoline, plastics, synthetic rubbers, dyes, and industrial solvents. It is also the parent compound for a large number of other aromatic compounds.
Toxicity of benzene hexachloride
- When it comes into touch with the skin, eyes, or other organs, it is extremely hazardous.
- It has a devastating effect on the neurological system and may result in the death of the individual who has been exposed to benzene hexachloride poisoning.
- Lindane poisoning can be severe and have long-term effects on the neurological system.
Uses
- Because benzene hexachloride is a weedicide, it is also a fertilizer.
- Domestic cockroaches are controlled using benzene hexachloride.
- Gamma benzene hexachloride, or limited, is an insecticide.
- It can be found in various lice treatment creams and shampoos.
- It finds use in the pharmaceutical business.
More Topics
Combustion Reactions| Introduction, Reaction, & Facts
How Many Cups in a Gallon? Cups to Pints, Quarts, and More
Acidic Hydrogen
Is NH3 Acid or Base?| NH3 Properties
Ammonium Nitrate
How cold is Liquid Nitrogen?
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



