Intermolecular forces are the forces of attraction that exist between atoms, molecules, and ions when they are in close proximity to one another. Intermolecular forces are not to be confused with intramolecular forces, which refer to covalent bonding within molecules.
There are three types of intermolecular forces:
- London dispersion forces
- Dipole-dipole forces
- Hydrogen bonding
Table of Contents
London Dispersion Forces
London dispersive forces result from the creation of temporary instantaneous polarities across a molecule caused by electron circulations. They exist for all substances, whether polar or nonpolar in nature.
Such forces are observed in nonpolar compounds such as halogens: fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2).
Dipole-Dipole Interations
Dipole-dipole forces are attractive forces that exist between the positive and negative ends of polar molecules. They are significantly weaker than intramolecular forces (ionic or covalent bonds) and only have an effect when the molecules involved are close together.
Hydrogen Bonding
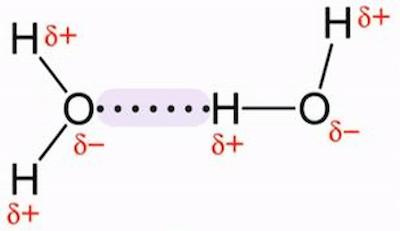
A hydrogen bond is a form of intermolecular force that occurs when a hydrogen (H) atom is bonded to a strongly electronegative atom (fluorine, nitrogen, or oxygen) and resides near another electronegative atom with a lone pair of electrons.
Related Links
Sigma Bond- Definition & Explanation
CH4 Polarity| Simple Explanation
Charge of Ammonia (NH3)| Simple Steps
Molar Mass of Methanol| Easy-Explanation
HCL Intermolecular Forces
CH4 Intermolecular Forces| Simple Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023