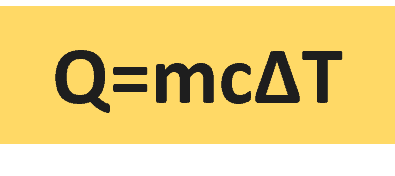The amount of heat required to increase the temperature of a one-kilogram mass of a substance by one Kelvin is referred to as specific heat (cp).
The specific heat formula is Q/m ΔT = cp, where Q is the amount of heat supplied to the specimen and ΔT is the rise in temperature.
The value of cp depends on the nature of the substance.

| Specific heat definition | The amount of heat required to increase the temperature of the one-kilogram mass of a substance by one Kelvin temperature is referred to as specific heat (cp). |
| Specific heat of air constant pressure | 1.005 kJ kg-1 K-1 |
| Specific heat of air constant volume | 0.718 kJ kg-1 K-1 |
| Specific heat of water | 4.179 kJ kg-1 K-1 |
Table of Contents
Specific Heat Definition
The quantity of heat that must be added (or removed) from a unit mass of a substance to change its temperature by one degree Celsius is referred to as specific heat. It is a time-intensive property. To put it simply, identical masses of various substances need varying quantities of heat to elevate them over the same temperature interval.
Specific Heat Formula
Consider the temperature of a body of mass “m” changing by ΔT. A body’s heat (Q) absorption or release is proportional to its mass and temperature change.
Q ∝ mass of the body (m) ………(1)
Q ∝ change in temperature (ΔT) ………(2)
Combining equation 1 and equation 2
Q ∝ mΔT
Q = cp.m.ΔT
Where cp is the proportionality constant, it is determined by the nature of the material.
The formula demonstrates that the transmission of heat to an item is determined by two factors:
- The change in temperature
- The mass of the system
According to the formula, you must add twice the heat to create an equivalent temperature change in a doubled mass. Because a substance’s heating capacity varies with temperature and pressure, cp is usually calculated at a constant temperature and pressure, usually 25 degrees Celsius.
Specific Heat in Simple Terms
- Specific heat is the amount of energy required to raise the temperature of one unit of mass of a substance by one degree Celsius (or one Kelvin).
- It is a measure of how much heat energy a substance can absorb without significantly changing its temperature.
- Different materials have different specific heats, which depend on factors such as the chemical composition and physical structure of the material.
- Substances with higher specific heats require more energy to raise their temperature compared to those with lower specific heats.
- Specific heat is an important property in a variety of applications, from cooking and heating to designing thermal insulation and understanding the behaviour of materials in extreme environments.
Specific Heat Units
| Mass Units | Temperature Units | Energy Units | Specific Heat Units |
| kilogram | Celsius | Joule | J/kg°C |
| gram | Celsius | Joule | J/g°C |
| pound | Fahrenheit | BTU | BTU/lb°F |
| gram | Celsius | calorie | cal/g°C |
Note that the specific heat units can also be expressed using Kelvin instead of Celsius, as the change in temperature is the same regardless of which scale is used.
Real-Life Examples of Specific Heat
Real-life examples of specific heat are listed below
| No. | Field/Industry | Example |
| 1. | Cooking | Different foods have different specific heats, which affects cooking times and temperatures. For instance, water has a high specific heat, which means it takes longer to boil than oil. |
| 2. | Thermal insulation | Specific heat is important in designing effective insulation materials. Materials with low specific heat can absorb less heat energy, which means they can cool down more quickly and maintain a more stable temperature inside buildings. |
| 3. | Environmental science | Specific heat affects the behaviour of materials in extreme environments, such as the oceans and the atmosphere. For example, the specific heat of seawater is different from that of freshwater, which affects the way the oceans absorb and distribute heat energy. |
| 4. | Engineering | Specific heat is important in designing efficient and effective heat exchangers, refrigeration systems, and other engineering applications. Knowing the specific heat of different materials can help engineers determine how much heat energy is required to achieve a certain temperature change. |
Key Points
- It is the capacity of a substance that depends on the nature of the material.
- S.I unit is J/(kg⋅K) or J/(kg⋅C).
- It is an intensive property that depends only on the type and phase of a substance.
- cp is independent of mass or volume.
- As per the mathematical formula, constant (cp) = ΔQ if the temperature change of 1 kg mass is 1K.
- It is another physical property of matter.
- The cp of a gallon of milk is equal to the cp of a quart of milk.
- It does not depend upon the size of the object, unlike heat capacity.
- Water has an extremely high cp capacity, which makes it good for temperature regulation.
- The cp of copper (Cu) is 0.385 J/g °C. This means that 1 gram of Cu takes 0.385 joules of heat to raise its temperature by 1 degree Celsius.
cp of Water
Water has a cp of 4182 J/kg°C, which is more than that of any other common material. As a result, water is extremely vital for temperature regulation.
Water has a cp five times that of glass, which means it takes five times as much heat to increase the temperature of water by the same amount that it does for glass.
Specific Heat Formula-Sample Problem
A container has 2.5 liters of water at 20°C. What is the cp required to boil the water? The density of water is 1000 kgm-3 and the cp of water is 42000 J Kg-1 k-1
The volume of water is 2.5 liters and the mass of water is 2.5 kg.
Initial temperature T1 = 20 °C
Final temperature T2 = 100 °C
ΔT = T2-T1
= 100°C-20°C = 80°C or 80K
Q = cp.m.ΔT = 42000 x 2.5 x 80 = 840000J = 840 kJ
Summary
- Specific heat refers to the exact amount of heat needed to make one unit of mass of a substance one degree warmer.
- The specific heat formula is Q = m.ΔT.cp
- The SI unit of cp is joule per kilogram per kelvin
- The cp of water is 4182 J/kg°C
Related Topics
Room Temperature| Comfortable Temperature
Sublimation Definition| Explanation with Examples
What is Auxiliary Heat?
Carbon Footprint And Carbon Emissions
Frequently Asked Question
Some of the frequently asked questions are given below:
- What is specific heat, and how is it defined?
Answer: Specific heat is the amount of heat energy required to raise the temperature of one unit of mass (such as one gram or one kilogram) of a substance by one degree Celsius or Kelvin. It is defined as the ratio of the heat energy absorbed (Q) to the mass (m) and temperature change (ΔT), expressed as Q/mΔT. - How does the specific heat of water compare to that of other substances, and why is this important?
Answer: Water has a high specific heat compared to most other substances, which means it can absorb a lot of heat energy without a significant increase in temperature. This property is important because it allows water to act as a thermal buffer, helping to stabilize temperatures in both living organisms and the environment. - Why do some materials have higher specific heat than others?
Answer: Materials with higher specific heat require more heat energy to raise their temperature by a given amount because they have more internal energy “storage” capacity. This can be due to a variety of factors, such as the material’s atomic or molecular structure, the strength and type of intermolecular forces, and the ability of the material to expand or contract when heated or cooled. - How can specific heat be used to determine the heat energy absorbed or released by a substance?
Answer: Specific heat can be used in the following formula: Q = mcΔT, where Q is the heat energy absorbed or released by a substance, m is the mass of the substance, c is its specific heat, and ΔT is the change in temperature. This formula can be used to calculate the amount of heat energy involved in processes such as heating, cooling, and phase changes. - What are some real-life applications of specific heat, and how do they demonstrate its importance?
Answer: Some examples of real-life applications of specific heat include cooking, thermal insulation, environmental science, and engineering.
For example, knowing the specific heat of different foods can help chefs determine the best cooking times and temperatures, while the specific heat of insulation materials is important for designing energy-efficient buildings. Understanding the specific heat of seawater can help scientists model ocean currents and weather patterns, while engineers can use specific heat data to design heat exchangers, refrigeration systems, and other thermal management systems.
The specific heat formula is the amount of heat supplied to the specimen, divided by the resulting temperature increase. cp is related to the unit mass of the specimen. The mathematical form of the formula is Q/m ΔT = cp where Q refers to the amount of heat supplied to the specimen and ΔT is the rise in temperature.
More Interesting Topics
Hess’s Law: Statement, Equation, & Examples
Biot Savart Law: Statement, Derivation & Applications
Condensation Reaction| Easy Explanation
Sublimation Examples| Process & Case Study
Malus Law-Overview and Examples
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023