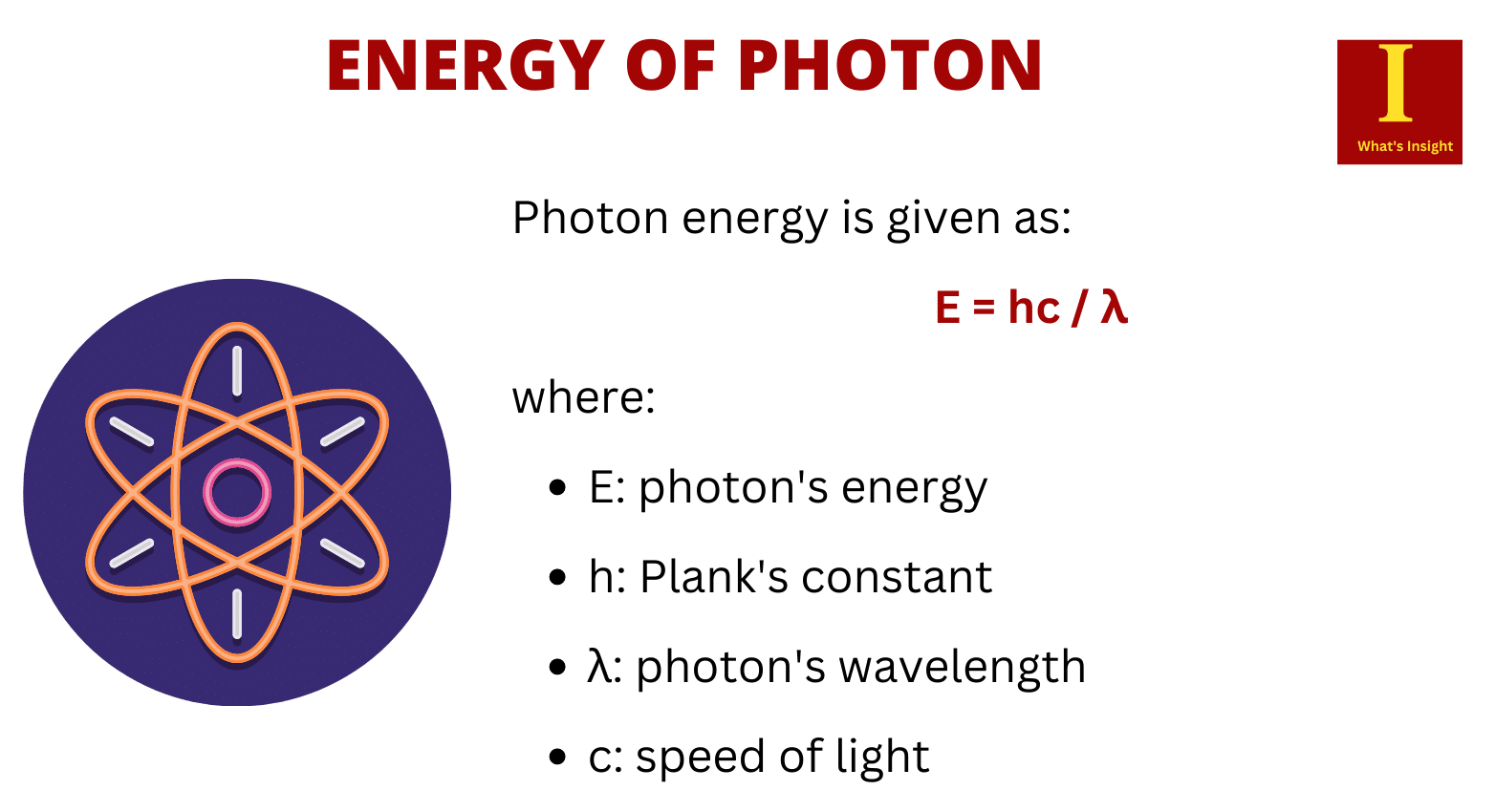
The energy of a photon is related to its frequency and its wavelength. It is directly proportional to frequency and inversely proportional to wavelength. The energy associated with a single photon is given by
E = hν = hc/λ
where
- E = energy
- h = Planck’s constant [6.626 x 10-34 J·s]
- ν = frequency [SI units of s–1 or Hertz, Hz]
- c = the speed of light [2.998 x 108 m s–1]
- λ = wavelength [meters]
E = hc/λ = [6.626 x 10-34 J·s x 2.998 x 108 m s–1]/λ
= 1.986× 10−16 J nm photon−1/λ
Photon wavelength: 0.377 um or 377 nm
Photon energy (E) = 3.2883eV.
The shorter the wavelength, the more energetic the photon, the longer the wavelength, the less energy the photon.
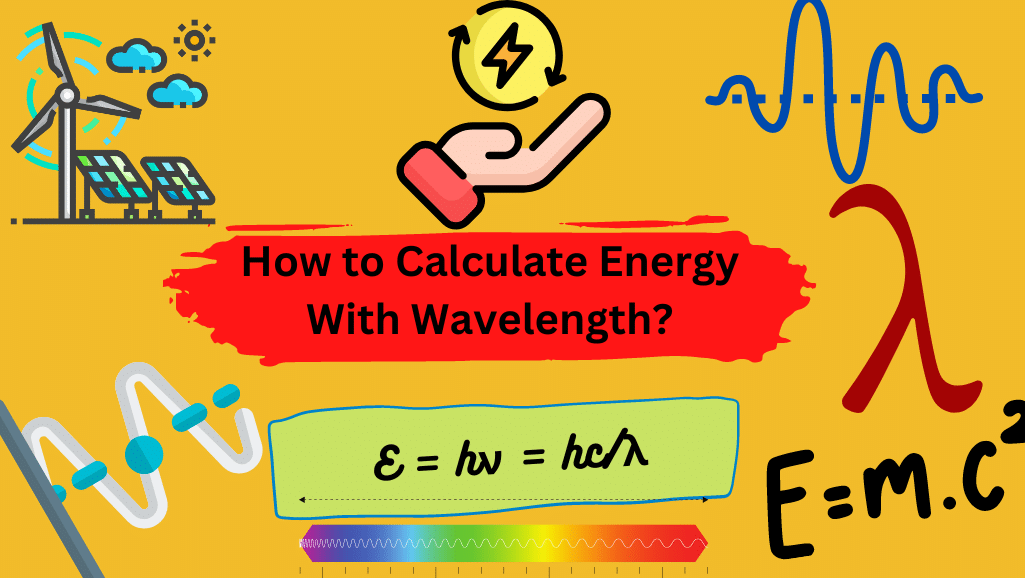
Energy Calculations with wavelength
The energy of red light
Red light wavelength (λ ) = 656.5 nm or 656.5 x 10-9 m
Speed of light c =2.998 x 108 m/s
E = h * c/λ = [6.63 x 10-34 J*s x 2.998 x 108 m/s]/ 656.5 x 10-9 m
Energy of red light = 3.03 x 10-19 Joules
The energy of green light
wavelength of green light = 562nm
E = h * c/λ = [6.63 x 10-34 J*s x 2.998 x 108 m/s]/ 562 x 10-9 m
The energy of green light is 3.537∗10−19 Joules
More Interesting Topics
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023