The photoelectric effect is a phenomenon that occurs when light strikes a metallic surface and causes electrons to eject from it. The electrons emitted in this process are photoelectrons.

Alkali metals are commonly used in the photoelectric emission process. These are lithium, sodium, potassium, rubidium, and cesium, which exhibit a photoelectric effect when exposed to visible light. The Photoelectric effect equation is given as:
E = hf = Φ + ½ mv2max
Where:
- h = Planck’s constant (J s)
- f = the frequency of the incident radiation (Hz)
- Φ = the work function of the material (J)
- ½ mv2max = Ek(max) = the maximum kinetic energy of the photoelectrons (J)
- Ek(max) depends only on the frequency of the incident photon, and not the intensity of the radiation
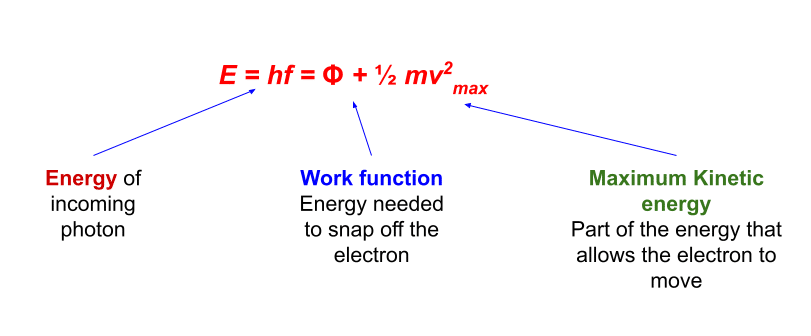
Table of Contents
Photoelectric Effect Facts
- According to Planck’s formula, light is a beam of particles whose energies are related to their frequencies. The photons collide with the atoms when that beam is directed at a metal. If the frequency of a photon is high enough to knock an electron off, the collision produces the photoelectric effect.
- A photon’s energy is equal to hf.
- This energy is transferred to the electron in order to free it from a material (the work function), and the remainder is given to the emitted photoelectron as kinetic energy.
- hf0 = Φ, where f0 = threshold frequency, photoelectric emission only just occurs

Photoelectric Effect in Simple Words
- The photoelectric effect is a phenomenon where electrons are ejected from a metal surface when it is exposed to light.
- The formula for the photoelectric effect is given by KE = hf – Φ.
- KE represents the kinetic energy of the emitted electron.
- h is the Planck constant.
- f is the frequency of the incident light.
- Φ is the work function of the metal, which is the minimum amount of energy needed to remove an electron from the metal.
- The photoelectric formula shows that the kinetic energy of the emitted electron depends on the frequency of the incident light and the work function of the metal.
- If the frequency of the incident light is below a certain threshold frequency, no electrons are emitted regardless of the intensity of the light.
- The photoelectric formula is an important part of the study of quantum mechanics and has many practical applications, including in the design of solar cells and in the development of new materials for electronic devices.
Important Points of Photoelectric Effect
| Phenomenon | When electrons are ejected from a metal surface when it is exposed to light. |
| Formula | KE = hf – Φ where: KE is the kinetic energy of the emitted electron; h is the Planck constant, f is the frequency of the incident light; Φ is the work function of the metal. |
| Variables | KE represents the kinetic energy of the emitted electron; h is the Planck constant; f is the frequency of the incident light; Φ is the work function of the metal. |
| Threshold Frequency | If the frequency of the incident light is below a certain threshold frequency, no electrons are emitted regardless of the intensity of the light. |
| Practical Applications | The photoelectric effect has many practical applications, including in the design of solar cells and in the development of new materials for electronic devices. |
Daily Life Examples of Photoelectric Effect
| Solar panels | Solar panels use the photoelectric effect to convert sunlight into electricity. When photons from the sun strike the surface of the solar panel, they eject electrons, which are then captured and used to generate electricity. |
| Digital cameras | Digital cameras use the photoelectric effect to capture images. Light enters the camera through a lens and strikes a sensor made up of tiny pixels. Each pixel contains a photodiode, which converts the light into an electrical charge. The charge is then measured and used to create a digital image. |
| Light sensors | Light sensors, such as those found in automatic lights or outdoor security cameras, use the photoelectric effect to detect light. When light strikes the sensor, it generates an electrical current that can be used to trigger a response, such as turning on a light or sounding an alarm. |
| Smoke detectors | Smoke detectors use the this principle to detect smoke. A light source inside the smoke detector sends a beam of light across a chamber. If smoke enters the chamber, it scatters the light, causing it to hit a light sensor and trigger an alarm. |
Summary
- The photoelectric effect is a phenomenon in which electrons are ejected from a metal surface when it is exposed to light.
- The effect is described by the equation KE = hf – Φ, where KE is the kinetic energy of the emitted electron, h is the Planck constant, f is the frequency of the incident light, and Φ is the work function of the metal.
- The photoelectric effect has many practical applications, including in solar cells, digital cameras, light sensors, smoke detectors, and remote controls.
More Links
| Planck’s Constant – Definition, Formula, and Applications | Quarks in Physics |
| Electromagnetic Force- Definition and Examples | Planck’s Constant – Definition, Formula, and Applications |
Latest posts by Umair Javaid, PhD Student (see all)
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023