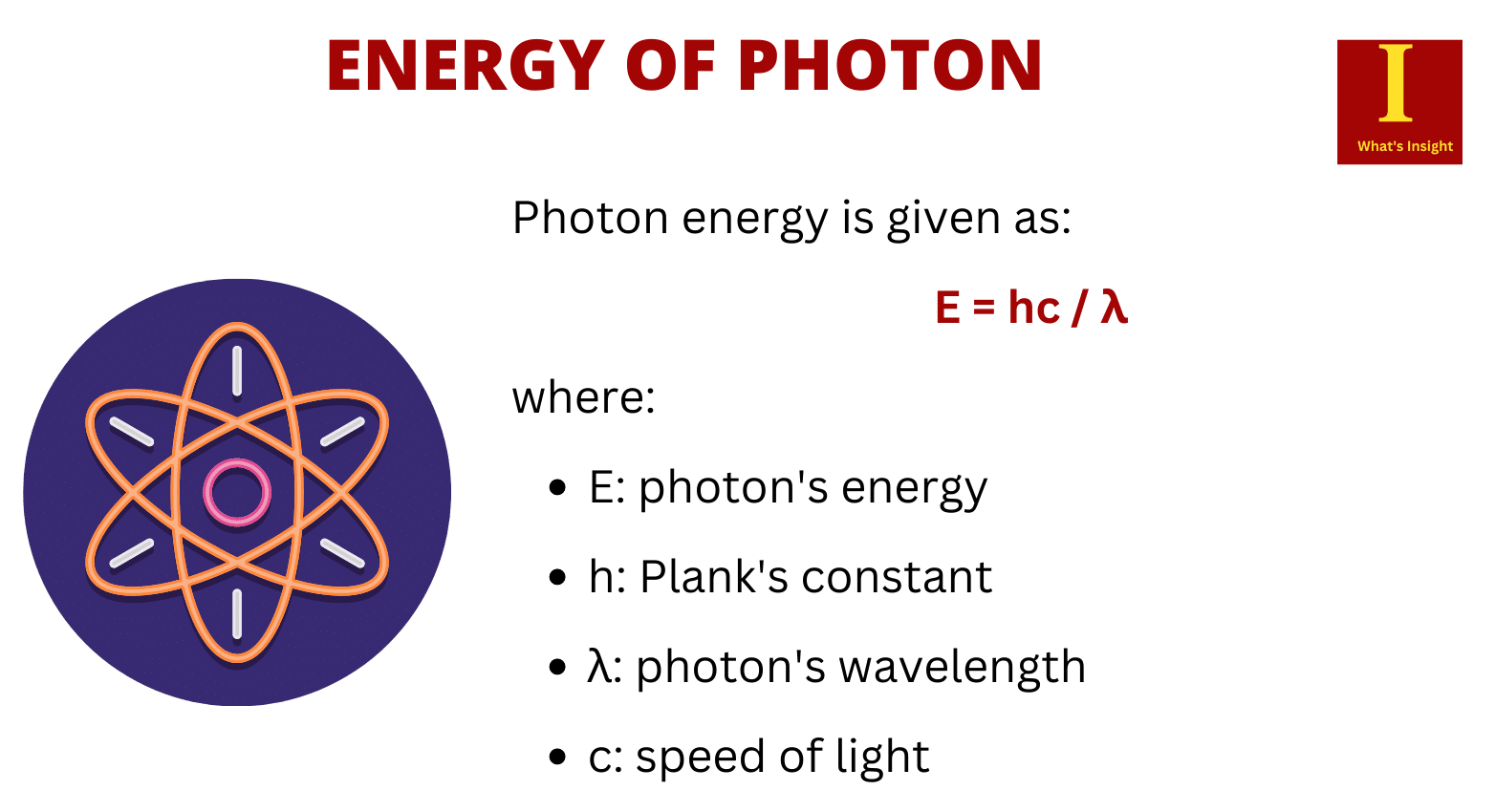
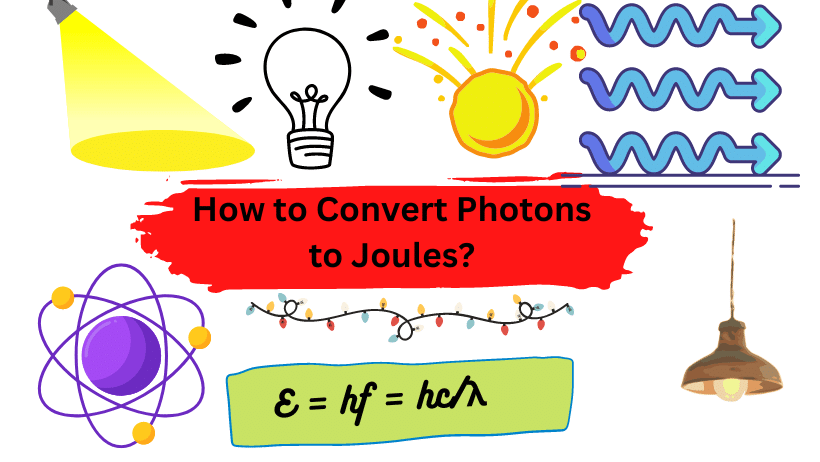
Photon energy is given as hν or = (h/2π)ω where h is Planck’s constant: 6.626 x 10-34 Joule-sec. Photon energy at one heartz frequency is 6.626 x 10-34 Joule. One photon of visible light contains about 10-19 Joules. To convert the energy of a photon to joule, use the following formula of energy of the photon
E = hf = hc/λ
Where,
- E = energy of the photon
- h = Planck’s constant (6.62607015) × 10-34 J.s or kg m2 s−1)
- f = photon frequency
- λ = photon wavelength
- c = speed of light (2.998 × 108 m/s)
Photon energy in joules at 1 Hz frequency is given as E= (6.62607015 × 10-34 J.s)(1 Hz) = 6.62607015 × 10-34 Joules.
The unit electron-volt (eV) is a more commonly used unit of energy than the joule (J). An electron volt is the amount of energy required to raise an electron through one volt, resulting in a photon with energy.
1 Joule = 6.24 × 1018 eV
E = hc/λ
hc = constant = 1.99 × 10-25 joules-m
1 eV for a photon is 1.602 × 10-19 J
hc = (1.99 × 10-25 joules-m) × (1ev/1.602 × 10-19 joules) = 1.24 × 10-6 eV-m
the units should µm (the units for λ)
hc = (1.24 × 10-6 eV-m) × (106 µm/ m) = 1.24 eV-µm
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023