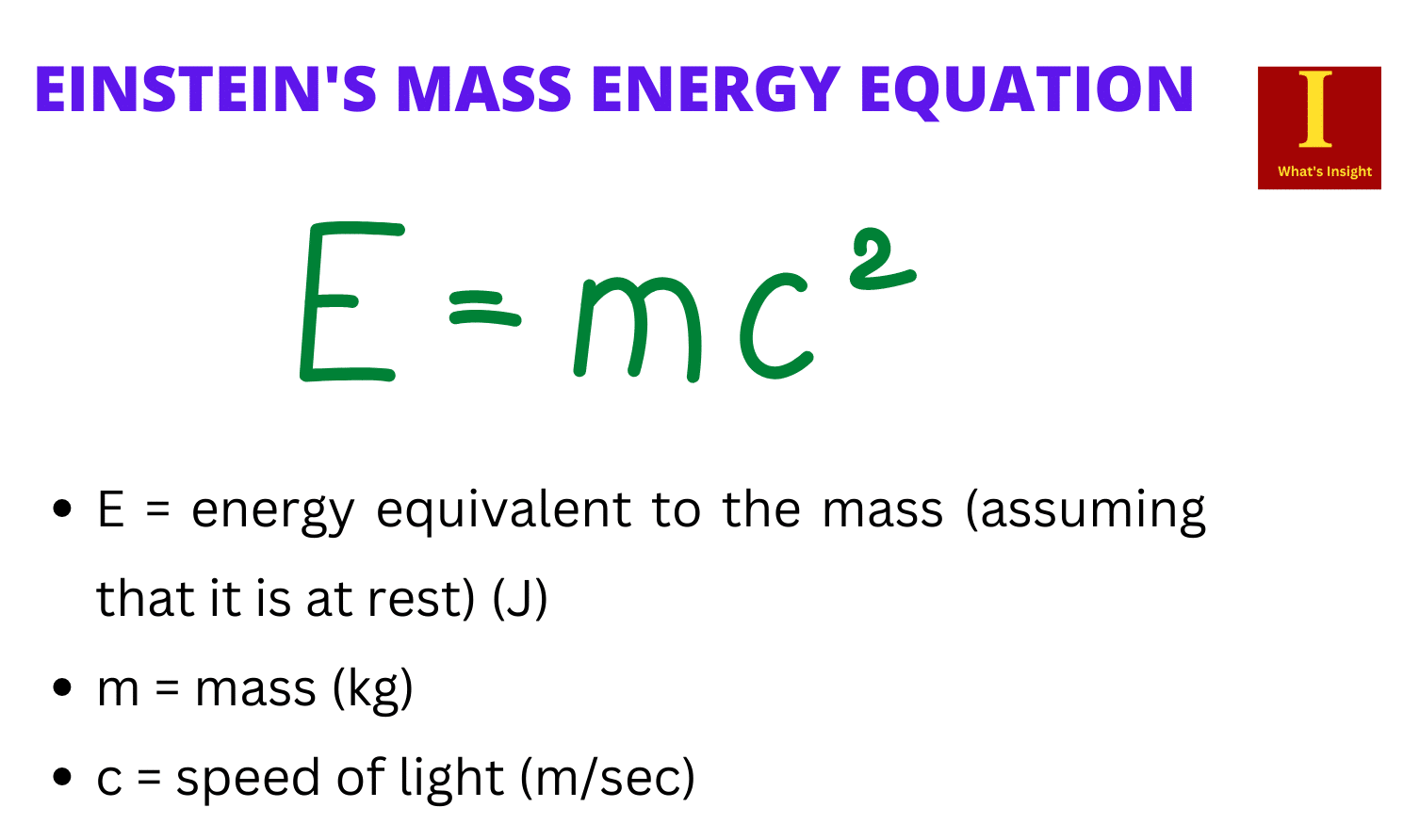Light is a type of energy that is emitted by a light source. Light is made up of photons, which travel at high speeds. A photon behaves both as a wave and as a particle. A photon’s behaviour is difficult to describe because it is both a wave and a particle. Scientists describe the amount of energy in a specific type of radiation in terms of its wavelength for convenience. Photons associated with different light frequencies have different energies and are used differently by ocean organisms.

Albert Einstein proposed in 1905 that light is composed of billions of small packets of energy known as photons. These photons have no mass, but each one has a unique amount of energy based on its frequency (number of vibrations per second). Each photon retains its wavelength. Photons with shorter wavelengths have more energy.
A photon is an elementary particle with energy that is proportional to its wavelength, which is inversely proportional to its energy. This means that the longer the photon’s wavelength, the lower its energy.
Photon energy is given as:
E = hc / λ
where:
- E: photon’s energy
- h: Plank’s constant
- λ: photon’s wavelength
- c: speed of light
Table of Contents
Photon Energy Formula- Real-Life Examples
- Light bulbs: When you turn on a light bulb, it emits photons that carry energy to produce light.
- Solar panels: Solar panels use photons from sunlight to create electricity.
- Remote control: When you press a button on a remote control, it emits a signal in the form of photons to change the channel or adjust the volume.
- Microwave ovens: Microwave ovens use photons to heat up food by exciting the water molecules inside it.
- X-rays: X-rays use high-energy photons to create images of bones and internal organs.
- Photosynthesis: Plants use photons from sunlight to create energy through the process of photosynthesis.
- Radio waves: Radio waves are a form of electromagnetic radiation that carries energy in the form of photons, and are used to transmit radio and television signals.
- Lasers: Lasers emit a concentrated beam of photons that can be used in many different applications, from cutting and welding to medical procedures.
Photon Energy Step-by-Step Derivation
- The energy of a photon can be expressed using Planck’s constant (h) and the frequency of the photon (f), according to the formula E = hf.
- Planck’s constant is a physical constant that relates the energy of a photon to its frequency and is approximately 6.626 x 10^-34 joule-seconds.
- The frequency of a photon is related to its wavelength (λ) by the formula f = c/λ, where c is the speed of light (approximately 3.0 x 10^8 meters per second).
- Substituting this expression for frequency into the formula for photon energy, we get E = hf = hc/λ.
- This expression tells us that the energy of a photon is inversely proportional to its wavelength.
In other words, photons with shorter wavelengths (such as X-rays and gamma rays) have higher energy than photons with longer wavelengths (such as radio waves). - We can calculate the energy of a photon in electron volts (eV) by dividing its energy in joules by the charge of an electron (approximately 1.602 x 10^-19 coulombs). This gives us the formula E(eV) = hc/λ / (1.602 x 10^-19).
- Finally, we can use this formula to calculate the energy of a photon given its wavelength, or vice versa, by rearranging the formula to solve for the unknown variable.
Overall, the derivation of photon energy involves using Planck’s constant, the frequency and wavelength of the photon, and simple algebraic manipulation to arrive at a formula that relates the energy of a photon to its physical properties.
Frequently Asked Questions
- What is the formula for the energy of a photon?
Answer: The formula for the energy of a photon is E = hf, where E is the energy, h is Planck’s constant, and f is the frequency of the photon.
- How is the energy of a photon related to its wavelength?
Answer: The energy of a photon is inversely proportional to its wavelength. In other words, photons with shorter wavelengths have higher energy than photons with longer wavelengths.
- What is Planck’s constant, and how is it used in the calculation of photon energy?
Answer: Planck’s constant is a physical constant that relates the energy of a photon to its frequency. It is used in the formula E = hf, where E is the energy, h is Planck’s constant, and f is the frequency of the photon.
- What is the unit of photon energy, and how can it be converted to electron volts?
Answer: The unit of photon energy is the joule (J). It can be converted to electron volts (eV) by dividing the energy in joules by the charge of an electron (approximately 1.602 x 10^-19 coulombs).
- How is photon energy used in everyday life?
Answer: Photon energy is used in a variety of ways in everyday life, including in light bulbs, solar panels, X-rays, microwave ovens, and lasers, among others. It is also essential to many scientific and technological applications.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023