Wavelength is defined as the distance between two successive crests or troughs. It is denoted by the sign λ(lambda). A wave is a regular and orderly disturbance that transmits energy from one location to another. The highest point of a wave is referred to as the crest, while the lowest point is referred to as the trough. For more on the wave properties please see the crest of a wave.
Table of Contents
Wavelength Formula
Light is defined based on its wavelength or frequency.
The velocity of light (v) is the product of its wavelength (λ ) and its frequency (f).
The wavelength of light = velocity of light/frequency of light.
λ = v/f
- λ = Photon wavelength, meters
- v = Velocity of light (c=299,792,458 m/sc)
- f = Frequency of the photon, Hz
Light may be considered as either wave or as discrete particles known as photons. The energy associated with a single photon is given by E = hf, where E is the energy (SI units of J), h is Planck’s constant (h = 6.626 x 10–34 J s), and f is the frequency of the radiation (SI units of s–1 or Hertz, Hz).
f=c/λ (c= velocity of light, 299,792,458 m/sec)
The energy of a single photon that has a wavelength λ is given by:
E = hc/λ= (6.626 x 10–34 J s)(c = 3.0 x 108 m/sec)/λ
By multiplying to get a single expression, hc = 1.99 × 10-25 joules-m
The electron-volt (eV) is a more often used unit of energy when dealing with “particles” such as photons or electrons than the joule (J). An electron volt is the amount of energy required to elevate an electron through one volt, resulting in a photon with one electron volt energy = 1.602 10-19 J.
hc = (1.99 × 10-25 joules-m) × (1ev/1.602 × 10-19 joules) = 1.24 × 10-6 eV-m
hc = (1.24 × 10-6 eV-m) × (106 µm/ m) = 1.24 eV-µm
E(eV)=1.24/(λµm)
Daily Life Examples of Wavelength
| Example | Wavelength Range | Application |
| Radio waves | 1 mm to 100 km | Radio and television broadcasting, communication (cell phones, Wi-Fi) |
| Microwaves | 1 mm to 1 m | Cooking food in microwave ovens |
| X-rays | 0.01 to 10 nm | Medical imaging |
| Ultraviolet light | 10 to 400 nm | Sunburns, tanning beds, water and surface disinfection |
| Visible light | 400 to 700 nm | Colour perception |
How wavelength is measured?
The wavelength of a wave is measured by measuring the distance between two successive crests or two adjacent troughs of the wave. Commonly, Wavelengths are measured in kilometres (km), meters, millimetres, micrometres (μm), and even smaller denominations, including nanometers, picometers (pm), and femtometers (fm).
Wavelength Symbol
Wavelength is usually denoted by the Greek letter lambda (λ); it is equal to the speed (v) of a wave train in a medium divided by its frequency (f): λ = v/f. [Reference]
Wavelength Concept in Simple Words
| Definition | Wavelength is the distance between two consecutive points on a wave that are in phase, meaning they are at the same point in their cycle. It is typically denoted by the symbol λ (lambda) and is measured in units of distance, such as meters or nanometers. |
| Properties | Wavelength is inversely proportional to frequency, meaning that as the frequency of a wave increases, its wavelength decreases. Wavelength is also related to the energy of a wave, with shorter wavelengths corresponding to higher energy waves, such as gamma rays and X-rays, and longer wavelengths corresponding to lower energy waves, such as radio waves and microwaves. |
| Applications | The concept of wavelength has many practical applications, including in the fields of optics, acoustics, and telecommunications. It is used to describe the properties of light, sound, and other types of waves, and is used to design and analyze a wide range of devices, such as lenses, microphones, and antennas. |
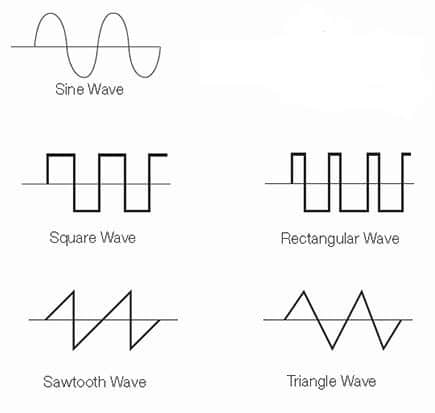
Waveforms
The most common periodic waveforms are sine, triangle, square, and sawtooth. These waveforms are called periodic because the wave they represent may be repeated to provide a continuous tone. The faster the wave repeats, the higher the pitch of the sound. The waveform affects the harmonics.
Energy of Light
Light is a kind of radiation that travels as electromagnetic waves. It may alternatively be represented as a flow of particle-like ‘wave-packets,’ known as photons, travelling at 300,000 kilometres per second.
The term energy of light refers to the energy of photons which is calculated by this equation, E = hf, where E is the energy of the photon in Joules; h is Planck’s constant, which is always 6.63 * 10-34 Joule seconds; and f is the frequency of the light in hertz.
Summary
- Wavelength is the distance between two consecutive points on a wave that are in phase.
- It is typically denoted by the symbol λ (lambda) and is measured in units of distance, such as meters or nanometers.
- Wavelength is inversely proportional to frequency, meaning that as the frequency of a wave increases, its wavelength decreases.
- Wavelength is also related to the energy of a wave, with shorter wavelengths corresponding to higher energy waves, such as gamma rays and X-rays, and longer wavelengths corresponding to lower energy waves, such as radio waves and microwaves.
- The wavelength formula can be expressed in two ways:
- λ = c / f, where λ is the wavelength, c is the speed of the wave, and f is the frequency of the wave.
- λ = h / p, where λ is the wavelength, h is the Planck constant, and p is the momentum of a particle.
Frequently Asked Questions
1. What is the wavelength of radio waves?
According to NASA, radio waves have the longest wavelengths in the electromagnetic spectrum, ranging from 0.04 inches (1 millimetre) to more than 62 miles (100 kilometres). They also have the lowest frequencies, ranging from roughly 3,000 cycles per second (3 kHz) to over 300 billion hertz (3 gigahertz).
2. What is gamma decay?
Gamma decay is the discharge of a gamma (γ) ray photon, a type of high-energy electromagnetic radiation, as a result of the radioactive decay of a nucleus. The energy spectrum is often in the 100 keV to 10 MeV range.
3. What are quarks?
In physics, quarks are the fundamental building units of matter. They’re most typically found in protons and neutrons, the fundamental components of the universe’s atoms. Based on present experimental findings, quarks appear to be really basic particles that cannot be further subdivided.
Solved Problems
- A sound wave has a frequency of 440 Hz and travels through air at a speed of 343 meters per second. What is the wavelength of this wave?
Answer: Using the formula wavelength = speed of wave/frequency of the wave, we get wavelength = 343 m/s / 440 Hz = 0.78 meters. - A radio wave has a wavelength of 2 meters. If the speed of light is 3 x 10^8 meters per second, what is the frequency of this wave?
Answer: Using the formula frequency = speed of light/wavelength, we get frequency = 3 x 10^8 m/s / 2 meters = 1.5 x 10^8 Hz. - A light wave has a frequency of 5 x 10^14 Hz. If the speed of light is 3 x 10^8 meters per second, what is the wavelength of this wave?
Answer: Using the formula wavelength = speed of wave/frequency of the wave, we get wavelength = 3 x 10^8 m/s / 5 x 10^14 Hz = 6 x 10^-7 meters (or 600 nanometers). - A guitar string has a length of 0.6 meters and is vibrating at its fundamental frequency. If the speed of sound in air is 343 meters per second, what is the wavelength of the sound wave produced by the guitar string?
Answer: Since the fundamental frequency of a guitar string is it’s first harmonic, the wavelength of the sound wave produced is twice the length of the string or 1.2 meters.
More Links
Longitudinal Waves| Physics
Transverse waves| Definition
Stationary Waves| Definition, and Properties
White Light & Working Principle of Prism
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023