Radioactive decay is a fascinating natural phenomenon that lies at the heart of nuclear physics. It involves the spontaneous transformation of unstable atomic nuclei into more stable configurations, accompanied by the emission of various types of radiation. This topic provides a comprehensive overview of radioactive decay, exploring its definition, mechanisms, and key concepts.
Table of Contents
Definition of Radioactive Decay
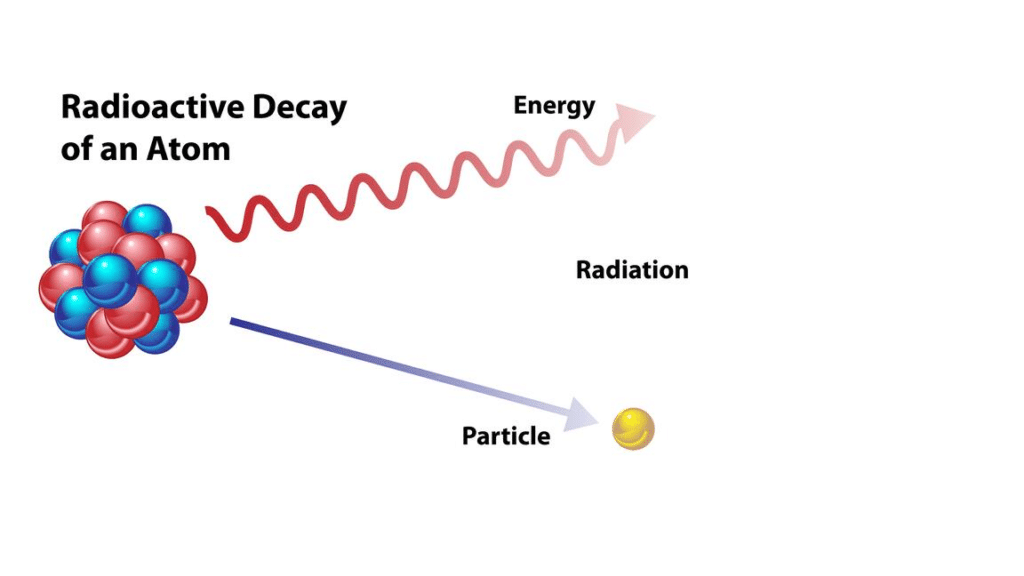
Radioactivity refers to the spontaneous emission of radiation from the nucleus of an atom. It is a natural process by which certain atomic nuclei become unstable and undergo transformations to attain a more stable state. These transformations involve the release of energy in the form of particles and/or electromagnetic waves.
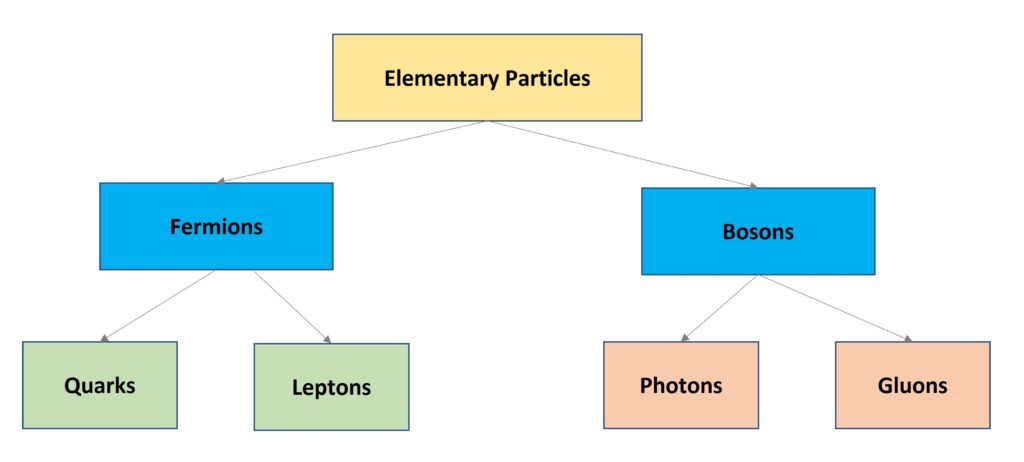
Types of Radiation:
Radioactive decay involves the emission of three main types of radiation:
- Alpha Particles (α): Alpha particles consist of two protons and two neutrons, making them identical to the helium-4 nucleus. They have a positive charge and relatively large mass. Due to their size and charge, they have low penetration power and can be stopped by a sheet of paper or a few centimeters of air.
- Beta Particles (β): Beta particles can be either electrons (β-) or positrons (β+). Beta-minus decay occurs when a neutron transforms into a proton, emitting an electron and an antineutrino. Beta-plus decay involves a proton transforming into a neutron, releasing a positron and a neutrino. Beta particles have a negative or positive charge, respectively, and are much smaller and lighter than alpha particles. They have moderate penetration power and can be stopped by a few millimeters of aluminum.
- Gamma Rays (γ): Gamma rays are electromagnetic waves, similar to X-rays and light, but with higher energy. They have no charge or mass and travel at the speed of light. Gamma rays are highly penetrating and require several centimeters of dense material, such as lead or concrete, to attenuate their intensity.
Emitted Radiation
The radiation emitted during radioactive decay can manifest in various forms, including the release of light energy, the emission of an alpha particle (consisting of two protons and two neutrons), or the expulsion of an electron or a positron. Additionally, there are numerous other subatomic particles that can be emitted.
To visualize the decay process, imagine a bowl representing an atom’s nucleus filled with green and purple grapes. In this analogy, each green grape represents a proton, while each purple grape represents a neutron. Let’s assume the bowl can hold exactly 40 grapes, symbolizing the nucleus of a calcium atom. Now, imagine attempting to add 22 purple grapes instead of the normal 20. Initially, you might be able to balance the extra grapes on top of the pile, but eventually, even a slight disturbance or bump to the side of the bowl will cause at least one of them to spill out.
The protons and neutrons within the nuclei of radioactive isotopes exhibit a similar instability. However, the decay of an unstable atom does not require an external force. The forces that hold protons and neutrons together inside the nucleus become unbalanced. Consequently, the atom seeks to achieve a state of balance. It accomplishes this by releasing energy and particles or by converting one or more neutrons into protons, which also releases energy. Numerous pathways exist for this decay process to occur. Nevertheless, the ultimate outcome remains consistent: the unstable isotope eventually transforms into a new, stable isotope.
More Links
Gamma Decay| Radioactive Decay
Difference between Heat and Radiation
Einstein’s Mass-Energy Equivalence Formula| E = mc²
Energy Definition and Examples| Easy Science
How Many Neutrons Does Hydrogen Have?
Relativistic Kinetic Energy| Easy Explanation
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023