Pressure definition: The force acting normally per unit area on the surface of a body is called Pressure. In other words, the pressure is force distributed over an area. Examples of pressure include air pressure in car tire support, sucking a drink through a straw, and flying aircraft. Pressure is a scalar quantity and Its unit is Pascal. Other units of pressure are listed below:
- Pascal (MKS system).
- N/m2 =1 Pa.
- bar = 105 Pa.
- atm =101325 Pa.
- Torr = 133.322 Pa.
- Foot-pound force/inch2 = 6894.76 Pa.
Table of Contents
Examples of Pressure
- Air pressure (psi) in a car tire supports the weight of the car.
- Fluid flows in a straw due to the difference in air pressure.
- In Aircraft, the air pressure difference between the top and bottom of the wing creates a force that lifts the wing up into the air.
- A bullet fired from a gun is driven by the sudden high pressure of generated gases.
- The balloon inflates because of the air pressure inside it.
- The pressure inside an inflated balloon.
Pressure Formula
The pressure formula is normal force per unit area.
Pressure = Force/Area
The pressure of an Ideal Gas
The ideal gas law states that the pressure of a gas multiplied by its volume equals the number of moles of gas multiplied by a constant (R) multiplied by the temperature of the gas. The ideal gas law is a crucial statement of the gas laws because it relates the amount of gas (moles) to its pressure, volume, and temperature.
Furthermore, the ideal gas law is a useful tool in chemical and engineering calculations that involve gases.
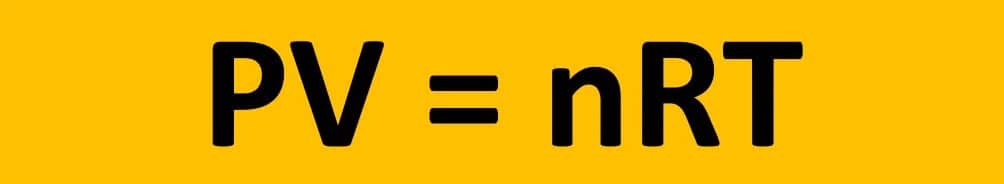
where:
- P is pressure
- V is volume
- n is the number of moles of gas
- T is the temperature (measured in kelvin)
- R is the ideal gas constant
Daily Life Examples of Pressure
Pressure is a force applied over an area and can be seen in many aspects of daily life. Here are some examples:
- Tyres in a car: The air pressure inside the tires of a car helps to support the weight of the vehicle and provides traction on the road.
- Cooking: When using a pressure cooker, the pressure inside the pot increases, cooking food faster and more efficiently.
- Blood pressure: Blood pressure is the pressure of the blood against the walls of the arteries. It is an important indicator of a person’s overall health and can be measured with a blood pressure monitor.
- Breathing: The pressure of air entering and leaving the lungs helps to regulate breathing and provide oxygen to the body.
- Water pressure: The pressure of water flowing through pipes helps to push it through the system and provides a steady supply of water to households and buildings.
- Atmospheric pressure: The pressure of the air around us affects weather patterns and can have an impact on our physical and mental well-being.
Liquid Pressure
Liquid Pressure (PL) is the pressure of a liquid on the surface of its container. It increases with the increase in the depth of liquid.
This pressure is more at the bottom as the liquid at lower depths has to support all of the water above it. Liquid pressure is a product of the density of liquid, gravity, and depth of liquid.

Pressure-Volume Diagram
The pressure-volume diagram (abbreviated as the PV diagram) is a graphical representation of pressure variations in a closed system. P-V diagrams can be used to calculate the efficiency of a system as well as the work done by or on the system.
The PV diagram is a curve that shows the fluctuation of the volume of material along the X-axis and the variation of pressure along the Y-axis. Each point on a PV diagram corresponds to a different state of the gas. The fundamental thermodynamic processes are isochoric, isobaric, and isothermal processes.
For details please refer to the full article “Pressure-Volume Diagram”.
Can Pressure Be Negative?
Pressure can be negative.
Pressure is usually positive, but negative pressure is also possible. Negative pressure signifies a partial vacuum, which is a gauge pressure measurement. Pull-on bulk liquids or solids also act as negative pressure.
What is Gauge pressure?
Gauge pressure is a pressure measurement in relation to the surrounding air pressure. It is, in other words, absolute pressure divided by atmospheric pressure. Gauge pressure can be positive or negative, but a positive or negative sign is usually omitted. Vacuum pressure is the pressure that is less than ambient pressure.
More Links
Static Pressure| Definition, Meaning, and Examples
Can Force be Negative?| Easy Explanation
Atmospheric Pressure| Definition & Examples
Can Work Be Negative?
Mechanical Energy Formula & Examples
Thermal Energy Equation- Simple Overview
Absolute Temperature Definition| Kelvin Scale
1MPa = 1000000 Pa = 1 x 106 Pa = 1 million Pa.
1 MPa = 145.038 psi.
1 MPa = 1000 kPa.
1MPa = 1000000 Pa
1 Pa =1/1000000 MPa
1 Pa = 10-6 MPa.
When an object is immersed in a liquid, then there are two forces act on the object.
Its weight acts in a downward direction.
An upthrust that acts in the upward direction.
1MPa = 1000000 Pa
100 MPa = 108 Pa.
1MPa = 1000000 Pa
1 Pa = 10-6 MPa.
101325 Pa =10.1325 MPa
The fan in a vacuum cleaner lowers the air pressure in its bucket. The atmospheric air rushes into it carrying dust and dirt with it through its intake port. The dust and dirt particles are blocked by the filter while the air escapes out.
When air is sucked through a straw, the air pressure within the straw decreases. This causes the atmospheric pressure to push the liquid up the straw.
The pressure of air in a car tire is 32 pis.
1 Pa = 0.000145038 psi (pounds/ square inch).
32 psi =220.632 kPa
Pressure on our body at sea level = 14.7 pounds per square inch.
Pressure increases as you go down under the ocean.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023