The process of vaporisation is the transformation of a liquid into a gas. The vaporisation process requires the use of energy in order for liquid particles to overcome intermolecular attractions and vaporise. This energy is referred to as the heat of vaporisation. The heat of vaporisation is different for each substance, but it is constant for each one.
The heat of vaporization formula is given as
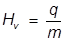
- Hv = heat of vaporization
- q = heat
- m = mass

More Links
Latest posts by Umair Javaid, PhD Student (see all)
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023