Heat transfer is the movement of thermal energy from a hot place to a cooler place due to a temperature difference. Heat can be transferred in three modes: conduction, convection and radiation. In simple words, Heat transfer refers to the process of transferring heat from a higher-temperature object (or system) to a lower-temperature object (or system). The general formula of heat transfer is given as:
Q = c × m × ΔT
Where,
- Q is the heat supplied to the system
- m is the mass of the system
- c is the specific heat capacity of the system
- ΔT is the change in temperature of the system
Some examples of heat transfer from everyday life are listed below:
- ultraviolet light from the sun (example of radiation)
- the heat from a stove burner
- When someone touches a hot object and feels the heat
- Boiling of water (convection)
- Working of heat radiators
- Hot air balloon (This heater heats up the air, which moves upwards)
- Melting of ice (example of convection)
- Working of cooking ovens
- Heating a pan on a stove (example of conduction)
- The light and heat emitted by an incandescent lamp (example of radiation)
Modes of Heat Transfer
There are three modes of heat transfer listed below with details included in the given table.
- Conduction
- Convection
- Radiation
| Conduction | Convection | Radiation | |
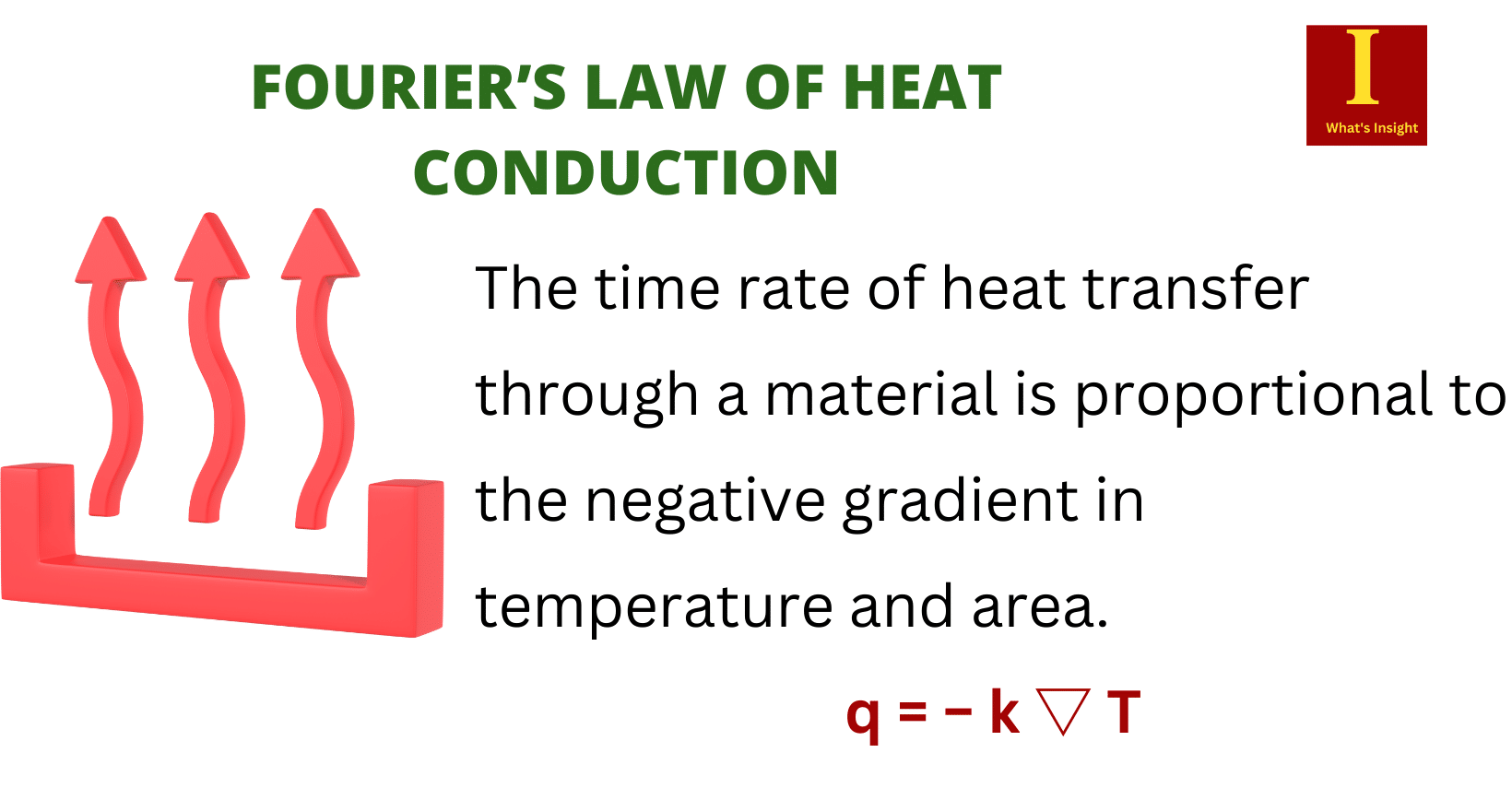
| Definition | Heat conduction is the transfer of energy within a homogeneous medium, such as a solid, liquid, or gas, caused by a temperature gradient. | Heat convection is the transfer of energy between a solid surface and a moving fluid, such as air or water. | Radiation is the transfer of energy between two or more bodies via electromagnetic waves due to temperature differences. |
| Formula | q=-k ΔT/L where ΔT = temperature difference; k = the thermal conductivity; L =thickness of the material. | q=hA(Ts-Ta) where Ts = temperature of the solid surface; Ta = the temperature of fluid away from the surface; h= heat transfer coefficient. | q = εσA (Th4-Tc4) where Th = absolute temperature of the hot body; Tc = absolute temperature of the surrounding; A= area of the object; σ = Stefan–Boltzmann constant (5.67×10-8 W m-2 K-4); ε =emissivity of the surface. |
| Examples | – Ironing of clothes – Ice cooling down your hand | – Boiling of water – Hot air rising, cooling, and falling (convection currents) | – sunlight heats the ground – microwaves from an oven |
More Links
Latest posts by Umair Javaid, PhD Student (see all)
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023