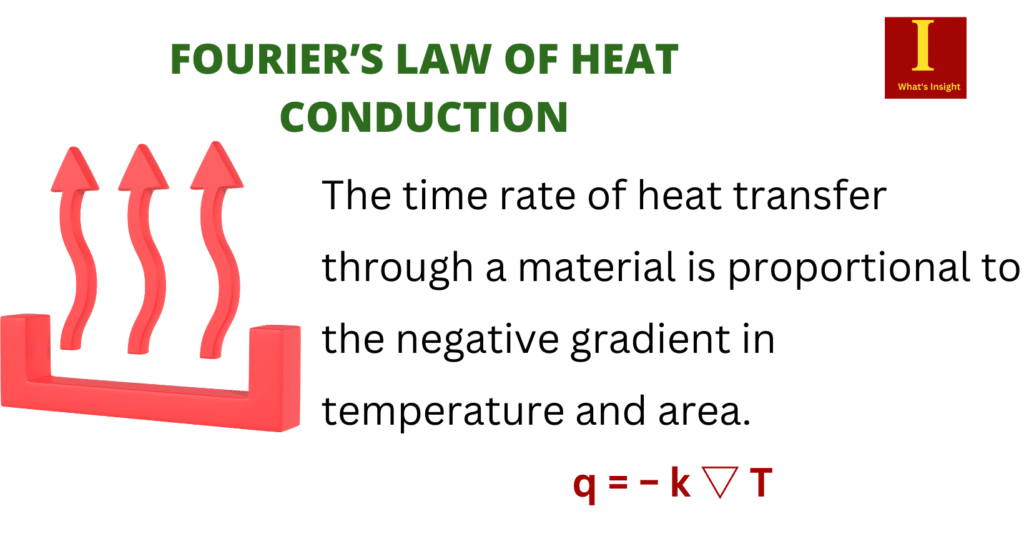
Fourier’s law of thermal conduction helps us in quantifying heat transfer processes using appropriate rate equations. According to Fourier’s law of thermal conduction, the time rate of heat transfer through a material is proportional to the negative gradient in temperature and area. Its differential form is:
q = − k ▽ T
Where:
- q is the local heat flux density (W.m. 2)
- k is the conductivity of the material (W.m-1)
- ▽ T is the temperature gradient (K.m-1)
- The proportionality constant (k) obtained from the relationship is known as the material’s thermal conductivity. A good thermal conductor has a high value of k and readily transfers energy by conduction.
Fourier’s Law of Thermal Conduction in Simple Terms
- Fourier’s Law of Thermal Conduction describes how heat energy is transferred through a material by conduction, which is the transfer of heat energy between particles that are in contact with each other.
- According to the law, the rate of heat transfer through a material is proportional to the temperature difference across the material and the thermal conductivity of the material, and inversely proportional to the distance the heat must travel.
- The equation for Fourier’s Law of Thermal Conduction is:
Q = -kA(dT/dx)
where Q is the heat flow rate;
k is the thermal conductivity of the material;
A is the cross-sectional area of the material;
dT is the temperature difference across the material;
dx is the distance the heat must travel. - The negative sign in the equation indicates that heat flows from regions of high temperature to regions of low temperature.
- The law applies to materials that conduct heat, such as metals, ceramics, and some polymers, but not to materials that are insulators, such as air and some plastics.
- Fourier’s Law of Thermal Conduction is important for understanding heat transfer in many everyday and industrial applications, such as building insulation, electronic cooling, and food processing.
Important Points
- Fourier’s law describes the relationship between the negative temperature vector gradient and the heat flux vector.
- The flow of heat through any material is referred to as thermal conductivity.
- The temperature difference, material thickness, and surface area all influence heat flow.
Real-Life Examples of Fourier’s Law of Thermal Conduction
Fourier’s Law of Thermal Conduction states that heat flows from a hotter object to a cooler object, and the rate of heat transfer is proportional to the temperature difference between the two objects and the thermal conductivity of the material in between them.
Here are some real-life examples of Fourier’s Law of Thermal Conduction:
- Cooking: When you cook food in a pan on a stove, the heat from the stove is conducted through the metal of the pan and into the food. The rate of heat transfer depends on the temperature difference between the stove and the pan, as well as the thermal conductivity of the metal.
- Home Insulation: Insulation in walls and attics is designed to slow the rate of heat transfer between the inside and outside of a house. This is done by using materials with low thermal conductivity, such as fibreglass or foam. The rate of heat transfer depends on the temperature difference between the inside and outside of the house.
- Car Engines: The cooling system in a car engine uses thermal conductivity to transfer heat away from the engine and into the surrounding air. This is done using a radiator, which is made of metal with high thermal conductivity, and a fan to help dissipate the heat.
- Electronic Devices: Electronics generate heat, and thermal conductivity is used to transfer that heat away from the device and into the surrounding air or a heatsink. The rate of heat transfer depends on the temperature difference between the device and the surroundings, as well as the thermal conductivity of the materials used.
- Earth’s Climate: The earth’s climate is influenced by the transfer of heat through the atmosphere and oceans. This transfer is driven by differences in temperature, which result in the movement of air and water currents. The rate of heat transfer depends on the thermal conductivity of the materials involved, as well as other factors such as wind and ocean currents.
Specific Unit Used for Thermal Conductivity
| No. | Unit | Description | Examples | Real-Life Examples (Approximate Values) |
| 1. | W/m-K | Watts per meter-kelvin | The common unit used in engineering for the thermal conductivity of materials | Heat transfer through walls, pipes, and other materials (0.1 – 10 W/m-K) |
| 2. | J/s-m-K | Joules per second-meter-kelvin | International System of Units (SI) unit for thermal conductivity | Heat transfer in electronic components, such as CPUs (100 – 1000 W/m-K) |
| 3. | Btu/hr-ft-°F | British thermal units per hour-foot-degree Fahrenheit | The common unit used in the United States for thermal conductivity | Heat transfer in building insulation (0.2 – 0.4 Btu/hr-ft-°F) |
| 4. | cal/s-cm-°C | Calories per second-centimetre-degree Celsius | The common unit used in some countries for thermal conductivity | Heat transfer in food processing, such as cooking or refrigeration (0.2 – 1.5 cal/s-cm-°C) |
| 5. | erg/s-cm-°C | Ergs per second-centimetre-degree Celsius | The common unit used in some scientific fields for thermal conductivity | Heat transfer in microscale systems, such as microchips (1 – 10 erg/s-cm-°C) |
Limitations of Fourier’s Law of Thermal Conduction
Fourier’s Law of Thermal Conduction assumes that the temperature and thermal conductivity are constant over time and distance. In reality, temperature and thermal conductivity can vary with time and distance, and other factors such as radiation and convection can also affect heat transfer.
Frequently Asked Questions
- What is Fourier’s Law of Thermal Conduction?
- Fourier’s Law of Thermal Conduction states that the rate of heat transfer between two objects is proportional to the temperature difference between them and the thermal conductivity of the material in between them.
- What is thermal conductivity?
- Thermal conductivity is the ability of a material to conduct heat. It is measured in watts per meter-kelvin (W/m-K).
- How does temperature difference affect the rate of heat transfer?
- The greater the temperature difference between two objects, the faster heat will flow from the hotter object to the cooler object.
- How does thermal conductivity affect the rate of heat transfer?
- The higher the thermal conductivity of the material between two objects, the faster heat will flow between them.
- What materials have high thermal conductivity?
- Metals such as copper, aluminium, and silver have high thermal conductivity. Other materials such as diamond and graphite also have high thermal conductivity.
- What materials have low thermal conductivity?
- Materials such as air, foam, and fibreglass have low thermal conductivity. Insulating materials are often used to reduce heat transfer between two objects.
- What is the unit of thermal conductivity?
- The unit of thermal conductivity is watts per meter-kelvin (W/m-K).
- What are some real-life examples of Fourier’s Law of Thermal Conduction?
- Examples include cooking, home insulation, car engines, electronic devices, and the transfer of heat through the atmosphere and oceans in the earth’s climate.
- How can Fourier’s Law of Thermal Conduction be applied to engineering design?
- Engineers can use Fourier’s Law of Thermal Conduction to design more efficient heat transfer systems, such as cooling systems for electronics or insulation for buildings.
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023