The Gibbs free energy (G) of a system is defined as the system’s enthalpy minus the product of the system’s temperature (T) and entropy (S). . It is a state function because it is defined in terms of state functions, which are thermodynamic properties. Its mathematical form is given as G = H – TS.
State functions are the values which depend on the state of the substance like temperature, pressure or the amount or type of the substance. Gibbs free energy is used to predict whether a chemical process is spontaneous or non-spontaneous. Gibb’s free energy is also called free energy.

In technical terms, Gibbs free energy is the quantity of energy associated with a chemical reaction that can be used to do work and is the sum of its enthalpy (H) and the product of the temperature and the entropy (S) of the system. This quantity is defined as follows:
G = H – TS
or
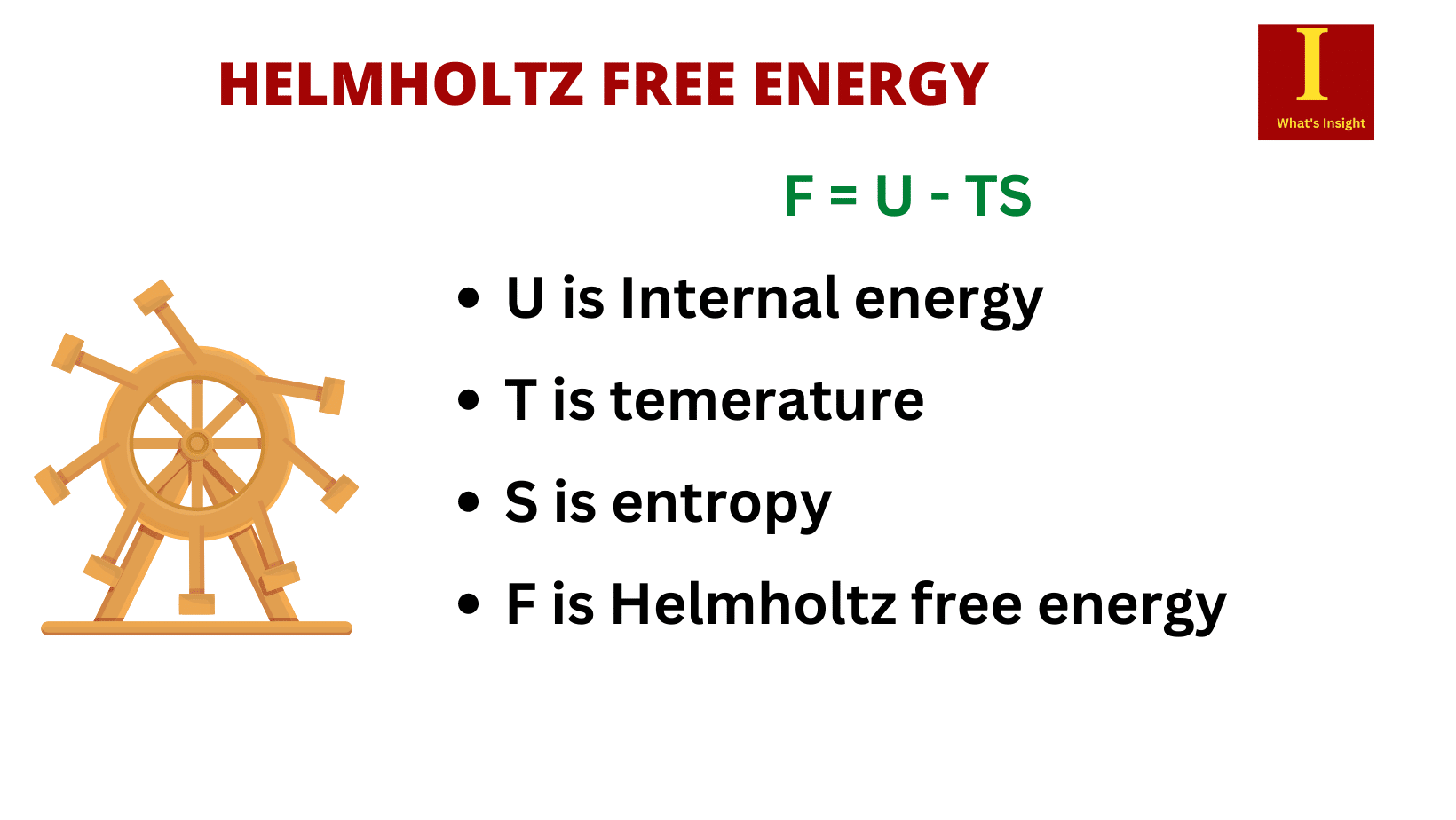
G = U +PV – TS
Where
- T is the temperature (K)
- S is entropy ( joule/kelvin)
- H is the enthalpy (joule)
- U is the internal energy (joule)
- P is pressure (pascal)
- V is the volume (m3)
Table of Contents
Gibbs Free Energy Formula in Simple Words
- The Gibbs free energy formula is G = H – TS.
- G represents the Gibbs free energy.
- H is the enthalpy (total heat content) of the system.
- T is the temperature in Kelvin.
- S is the entropy (degree of disorder) of the system.
- The formula tells us how much energy is available to do useful work in a chemical reaction or other thermodynamic process.
- If the value of G is negative, the reaction is spontaneous and can proceed without the addition of energy.
- If the value of G is positive, the reaction is non-spontaneous and requires an input of energy to occur.
- If the value of G is zero, the reaction is at equilibrium and is neither spontaneous nor non-spontaneous.
Daily Life Examples of Gibbs Free Energy
| Combustion | When a fuel such as wood or gasoline is burned, it undergoes a chemical reaction that releases energy. The Gibbs free energy of the reactants and products can be calculated to determine whether the reaction is spontaneous or requires an input of energy. |
| Batteries | Batteries convert chemical energy into electrical energy through a redox reaction. The Gibbs free energy change of the reaction can be used to determine the voltage of the battery. |
| Metabolism | The breakdown of food molecules in our bodies is a complex series of chemical reactions that release energy. The Gibbs free energy change of each reaction can be calculated to understand how much energy is released and whether the reaction is spontaneous or requires energy input. |
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023