Carbon tetrachloride, often abbreviated as CCl4 or CCl₄, is a chemical compound composed of one carbon (C) atom bonded to four chlorine (Cl) atoms. In this comprehensive guide, we will unveil the Lewis structure of CCl4 and delve into its molecular shape, bond characteristics, properties, historical uses, and environmental considerations.
| Name of Molecule | Carbon Tetrachloride (CCl₄) |
| Bond Angles | approx. 109.5 degrees |
| Molecular Geometry of CCl₄ | Tetrahedral Geometry |
| No. of Valence Electrons | 32 |
| Hybridization | sp3 |
| Polarity | nonpolar |
| Bond Lengths | 1.78 Å |
Table of Contents
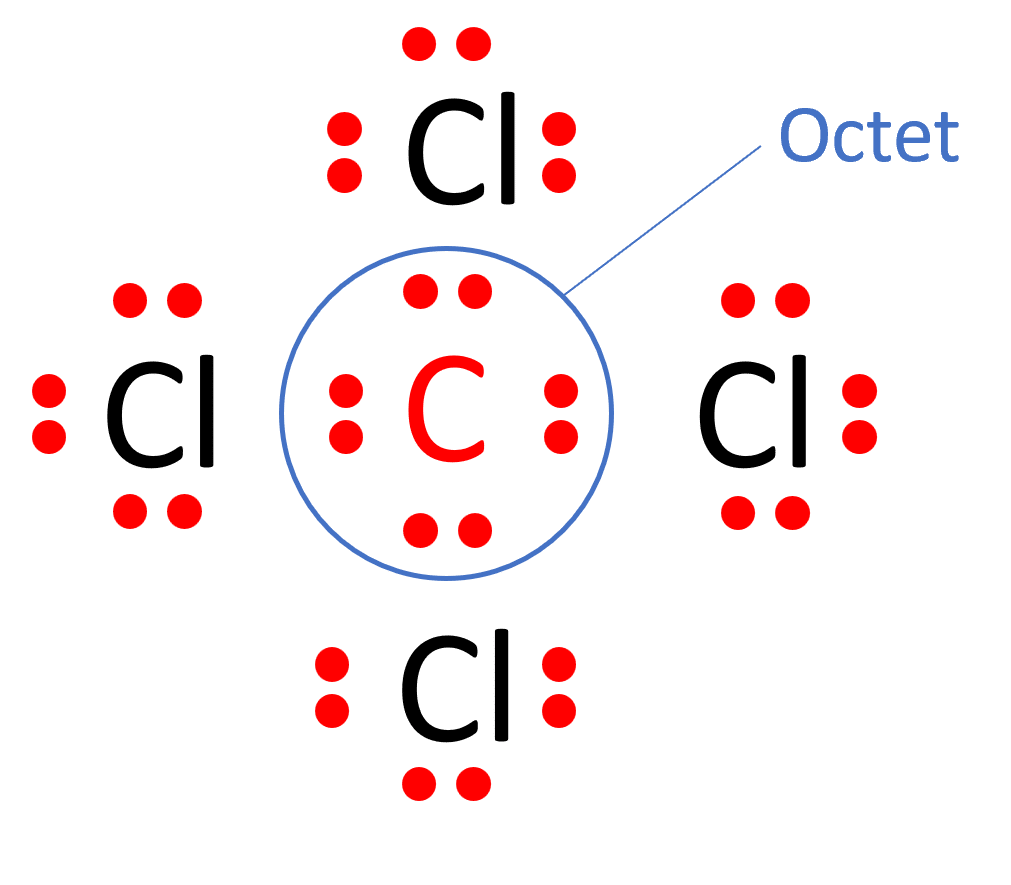
What is the Lewis Structure for CCl4 and How to Draw It:
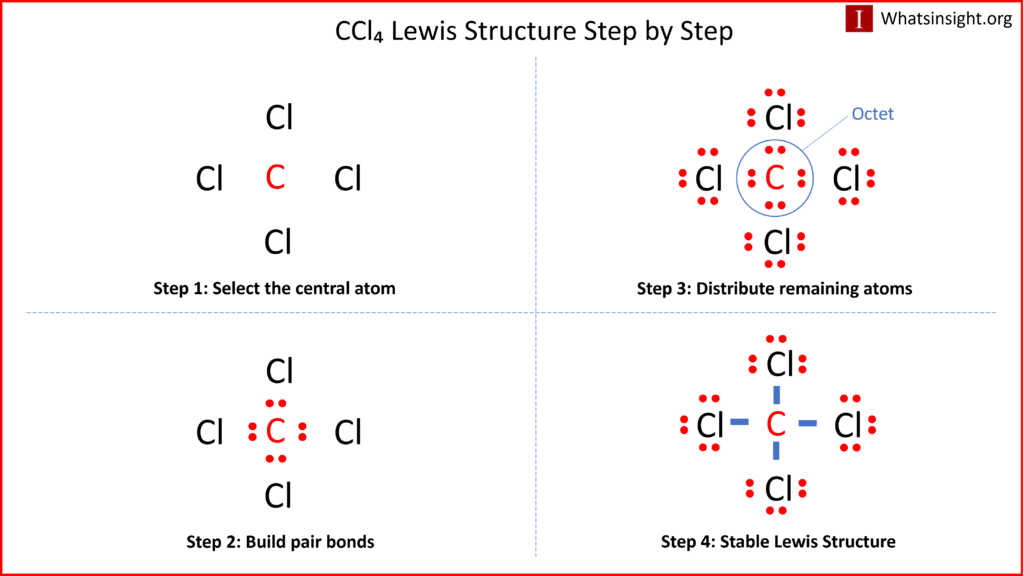
The Lewis structure of a molecule provides a visual representation of its atom arrangement and valence electrons. To draw the Lewis structure for CCl4, follow these steps:
a. Calculate the total number of valence electrons in CCl4: Carbon (C) has 4 valence electrons, and each chlorine (Cl) atom possesses 7 valence electrons. With four chlorine atoms in CCl4, the total valence electrons sum up to 4 (from carbon) + 7 x 4 (from chlorine) = 32 valence electrons.
b. Determine the central atom. In CCl4, carbon acts as the central atom because it is more electronegative and can form more bonds than chlorine.
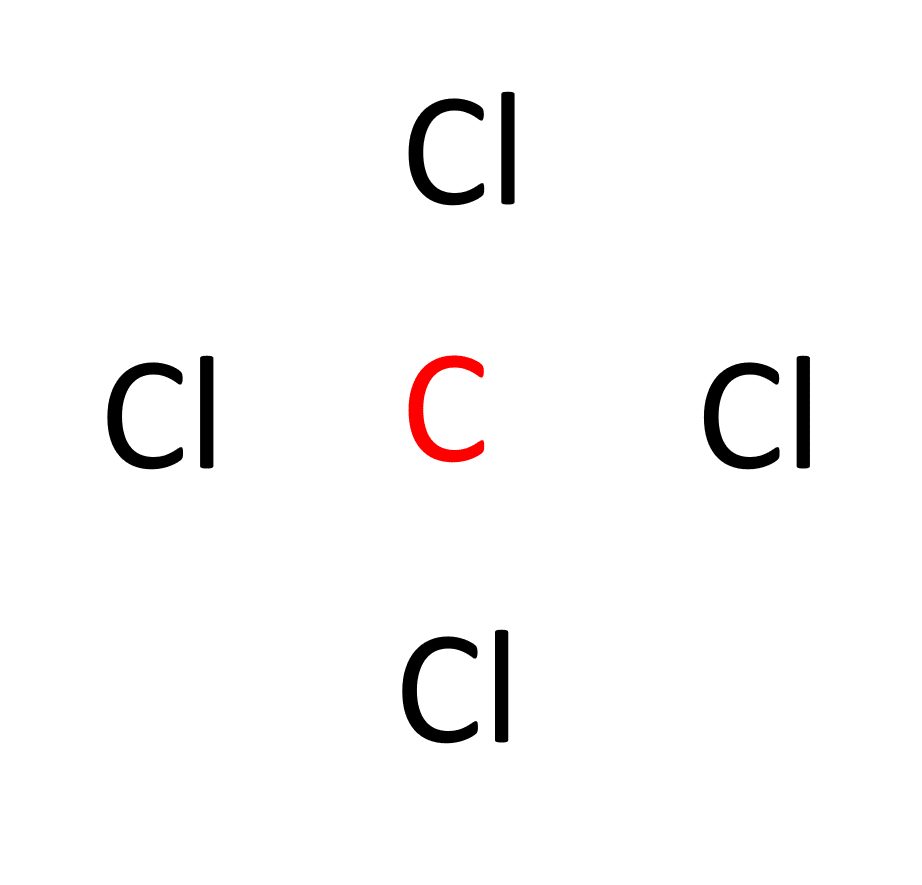
c. Establish single bonds connecting the atoms. Carbon forms single bonds with each of the four chlorine atoms (C-Cl).
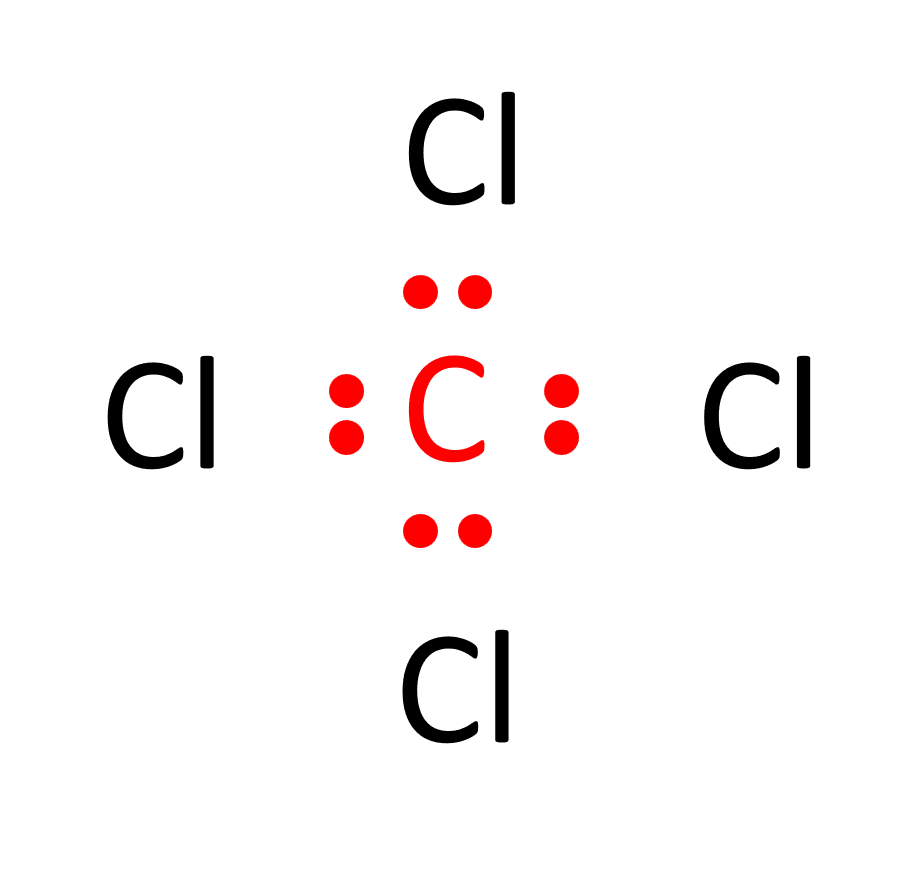
d. Distribute the remaining valence electrons to complete the octets of chlorine. Each chlorine atom achieves a full valence shell by forming a single bond with carbon, resulting in the Lewis structure of CCl4.
Molecular Shape of CCl4:
The molecular shape of CCl4 is tetrahedral. In this arrangement, the central carbon atom is bonded to four chlorine atoms, creating a symmetrical tetrahedral shape with bond angles of approximately 109.5 degrees.
Bond Type in CCl4:
In CCl4, carbon forms single bonds with each of the four chlorine atoms. These are sigma (σ) bonds, resulting from the overlap of atomic orbitals.
Properties of CCl4:
Carbon tetrachloride is a colorless, heavy, non-flammable liquid with a sweet odor. It has a high density and is relatively insoluble in water. Historically, CCl4 had a wide range of uses, including as a solvent, refrigerant, and fire extinguishing agent. However, its use has significantly diminished due to environmental and health concerns related to its toxicity and ozone-depleting potential.
Polar or Nonpolar Nature of CCl4:
CCl4 is a nonpolar molecule. While it contains polar C-Cl bonds due to the difference in electronegativity between carbon and chlorine, the symmetric tetrahedral molecular shape results in the cancellation of dipole moments, leading to a nonpolar molecule overall.
Hybridization in CCl4:
Carbon in CCl4 undergoes sp3 hybridization. This hybridization involves the combination of one s orbital and three p orbitals of carbon to form four sp3 hybrid orbitals. These hybrid orbitals overlap with the p orbitals of chlorine atoms, creating sigma bonds and maintaining the tetrahedral molecular shape of CCl4.
Conclusion
In conclusion, understanding the Lewis structure, molecular shape, bond type, and properties of CCl4 provides valuable insights into its chemical characteristics and behavior. The tetrahedral shape, single bonds, and nonpolar nature of CCl4 contribute to its unique properties. Moreover, its historical uses and environmental considerations highlight the evolving perspective on this compound in the realm of chemistry and industry.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023