Esterification definition: Esterification is the well-known equilibrium reaction of acids and alcohols to form esters, with various methods used to shift the equilibrium to the product.
Table of Contents
What are esters?
Esters are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an ester, the hydrogen in this group is replaced by a hydrocarbon group of some kind. In this article, We are focusing on the cases where hydrogen is replaced by an alkyl group, but it could also be an aryl group (one based on a benzene ring).
For instance, Ethyl ethanoate is the most commonly discussed ester. The hydrogen in the -COOH group has been replaced by an ethyl group in this case.
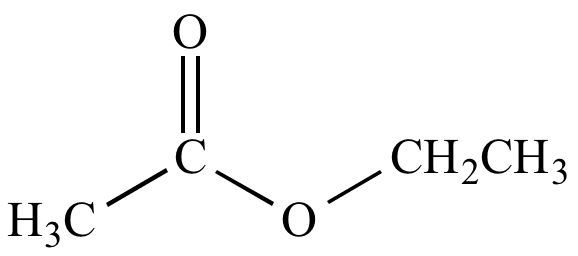
Some more examples of esters are as follows:
- Ethyl propanoate
- Proplyl methanoate
- Methyl butanoate
- Proplyl ethanoate
Preparing Esters from Carboxylic acids and Alcohols
Esters are formed when carboxylic acids and alcohols are heated in the presence of an acid catalyst. Typically, the catalyst is concentrated sulphuric acid. In some cases, dry hydrogen chloride gas is used, but this usually involves aromatic esters (ones containing a benzene ring).
Esterification is a slow and reversible reaction. The reaction between an acid RCOOH and an alcohol R’OH (where R and R’ can be the same or different) has the following equation:

More Links
Oxalic Acid Formula – (COOH)2
Molar Mass of Acetic Acid| Easy-Explanation
Condensation Reaction| Easy Explanation
Acetyl Group| 9 Key Points-Easy Explanation
Hydrogen Bond| Definition & Easy Explanation
Xenon Tetrafluoride (XeF4)-Chemical Compound
Neon Element| Properties & Uses
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023



