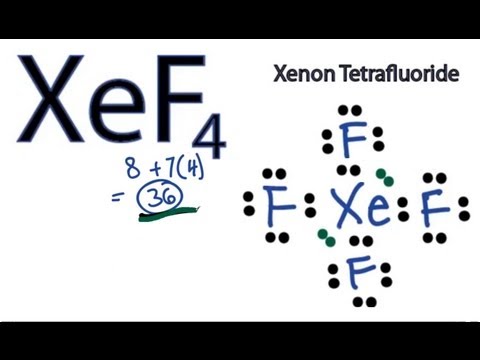
Xenon Tetrafluoride, represented as XeF4, is a fascinating chemical compound that has garnered significant attention in the world of chemistry due to its intriguing molecular structure and unique properties. In this article, we will embark on an in-depth exploration of the Lewis structure of XeF4, discussing its valence electrons, molecular geometry, hybridization, and polarity.
| Formula | XeF4 |
| Structure (Molecular Geometry) of XeF4 | Square Planar |
| Hybridization | sp3d2 |
| Molar Mass | 82.07 g/mol. |
| Polarity | nonpolar |
| Bond Angles | 90 degrees |
| Xe – F Bond Distance | 197 pm |
| No of Valence Electrons | 36 |
Table of Contents
Step-by-Step Construction of XeF4 Lewis Structure
Step 1: Counting Valence Electrons
Our journey into the Lewis structure of XeF4 commences with a fundamental task – determining the total number of valence electrons in the molecule. Xenon (Xe) is situated in Group 18 of the periodic table and boasts 8 valence electrons, while each fluorine (F) atom contributes 7 valence electrons. Given that there are four fluorine atoms in XeF4, the sum of valence electrons in the molecule stands at:
Valence Electrons in XeF4 = Valence Electrons in Xenon + Valence Electrons in Fluorine x 4
Valence Electrons in XeF4 = 8 + (7 x 4) = 36 valence electrons
Step 2: Determining the Central Atom
The pivotal choice in constructing the Lewis structure of XeF4 is selecting the central atom. Xenon (Xe) is our prime candidate for the central role, as it is less electronegative than fluorine. Consequently, Xenon assumes the central position, with fluorine atoms surrounding it.
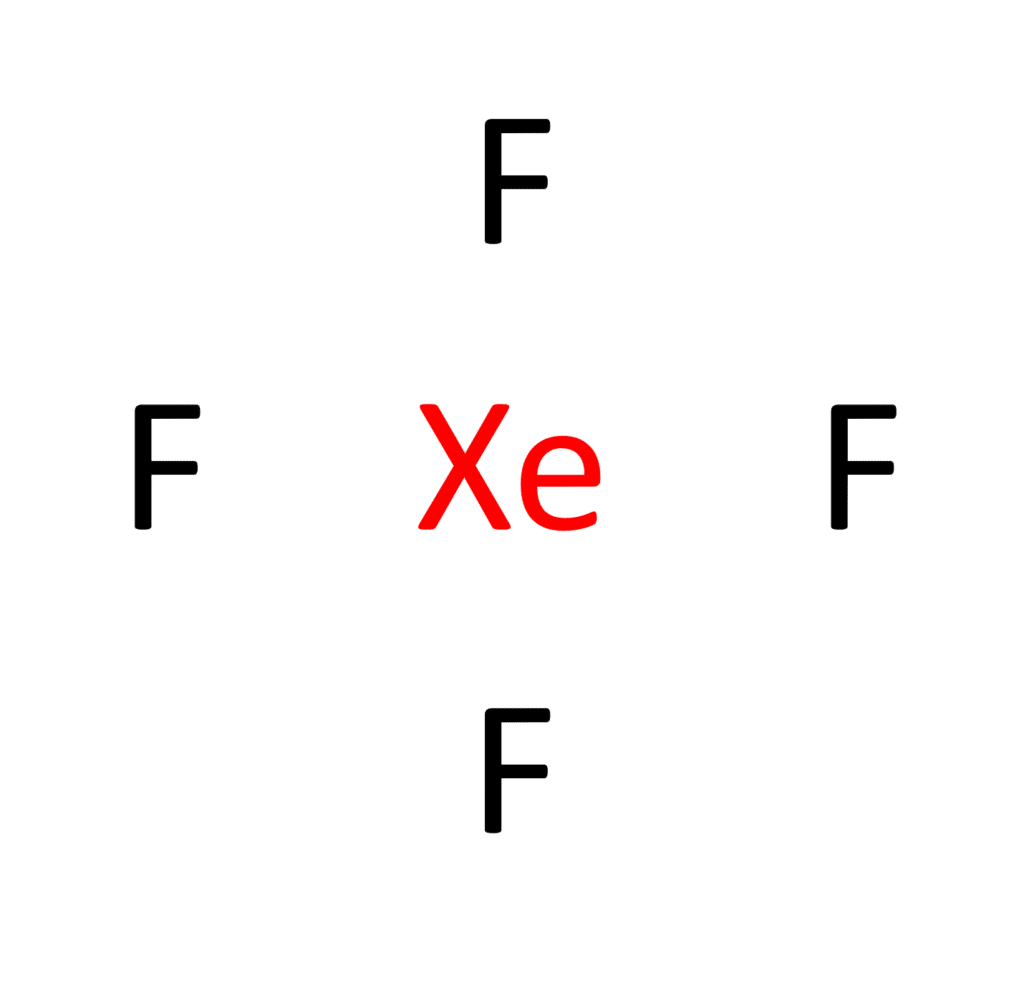
Step 3: Placing Electron Pairs Between the Atoms
With Xenon at the helm, it’s time to distribute the valence electrons around the atoms. Each fluorine atom will engage in a single bond with Xenon, utilizing 4 valence electrons. This initial bonding accounts for 16 of the 36 valence electrons, leaving us with 20 electrons to allocate.
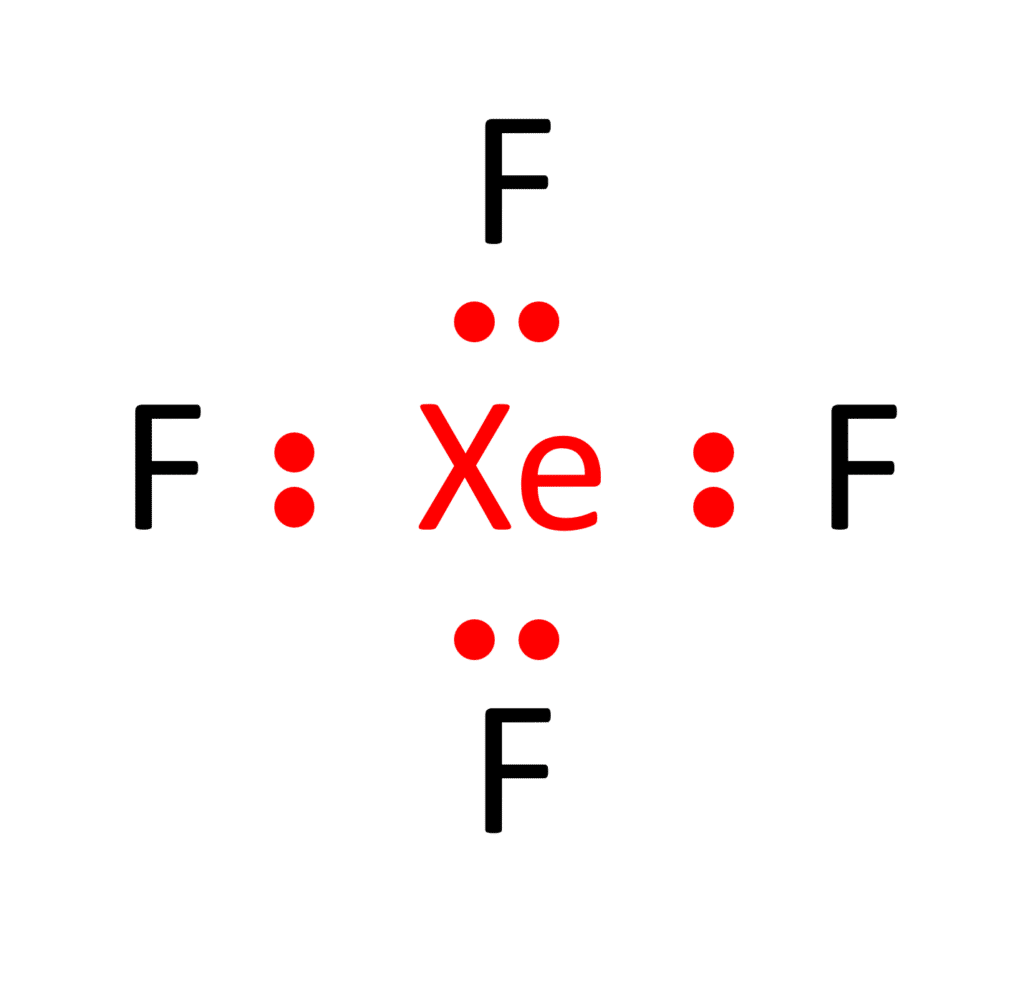
Step 4: Allocating the Remaining Electrons
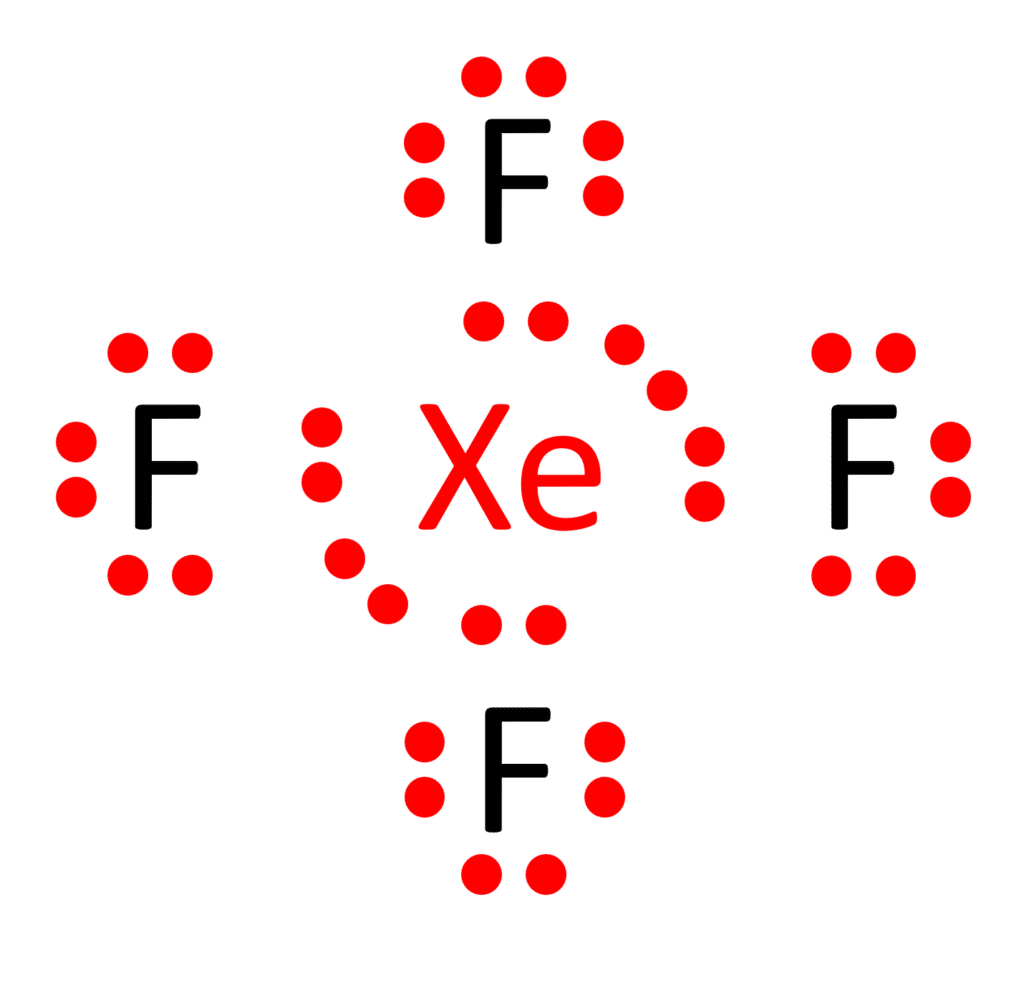
To bestow fluorine atoms with their octets and Xenon with its duet, we judiciously employ the remaining 20 valence electrons. Each fluorine atom’s octet is achieved by forming a single bond with Xenon. Consequently, Xenon attains its octet with 8 electrons around it.
The end result of our meticulous arrangement is the Lewis structure of XeF4, which portrays Xenon at the core, bonded singly to each of the four fluorine atoms, and all atoms enveloped by a complete complement of valence electrons.
Molecular Geometry of XeF4
The molecular geometry of XeF4 is characterized as square planar. This geometric configuration manifests when the four fluorine atoms and Xenon atoms collectively occupy the same plane, forming a square-like shape.
Hybridization of XeF4
The hybridization of Xenon in XeF4 is sp3d2. This intricate hybridization pattern arises from Xenon’s central role, its engagement in sigma bonding with four fluorine atoms, and the presence of two lone pairs.
Polarity of XeF4
XeF4 emerges as a polar molecule. While Xenon’s electronegativity is lower than that of fluorine, the existence of lone pairs on Xenon and the spatial arrangement of fluorine atoms in a square planar geometry result in an unequal distribution of charge. Consequently, XeF4 exhibits polarity, with partial positive and negative charges distributed across different regions of the molecule.
Conclusion
To sum it up, XeF4, or Xenon Tetrafluoride, unveils a captivating Lewis structure with Xenon assuming the central position, flanked by four fluorine atoms. Its molecular geometry assumes the form of square planar, and its unique polarity emerges from the strategic arrangement of atoms and the presence of lone pairs. A profound understanding of XeF4’s Lewis structure and properties is indispensable for comprehending its chemical behavior and reactivity.
Frequently Asked Questions (FAQs)
XeF4 adopts a square planar molecular geometry.
Yes, XeF4 is indeed a polar molecule, owing to its uneven charge distribution brought about by its molecular geometry and the presence of lone pairs on Xenon.
The hybridization of Xenon in XeF4 is sp3d2.
XeF4 is employed as a fluorinating agent in various organic synthesis processes and as a potent oxidizer in select chemical reactions.
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023