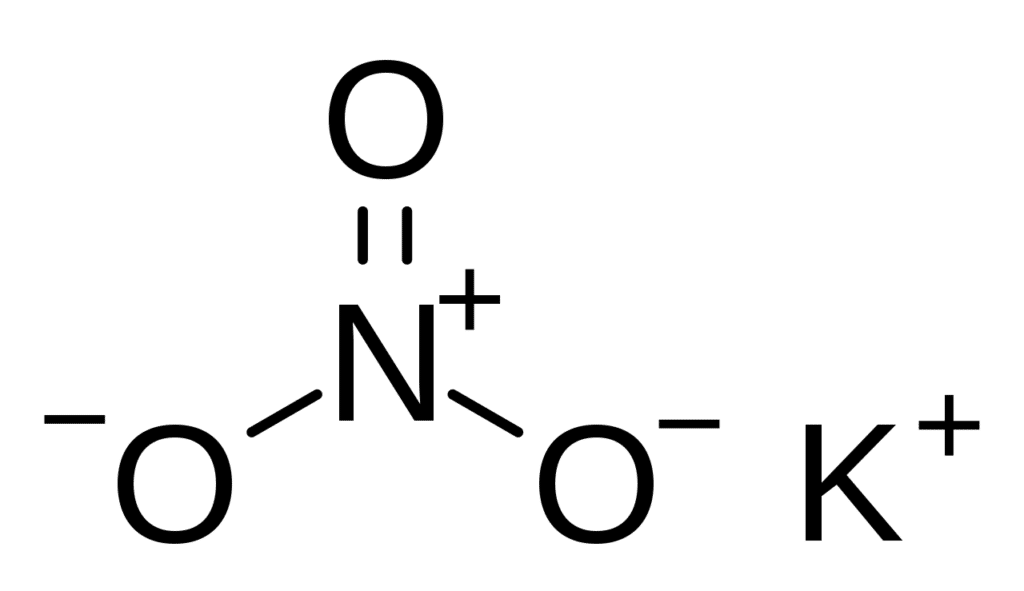
Saltpeter, also known as Potassium nitrate (KNO3) is a white crystalline solid that is commonly found as a powder. The majority of saltpeter (potassium nitrate) is created by a chemical reaction between nitric acid and potassium salts. It’s simple to produce it in the lab by reacting ammonium nitrate and potassium chloride in water.
KNO3 is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow.
| Compound Name | Saltpeter (Potassium nitrate) |
| Appearance | white in color with vitreous luster |
| Melting point | 334 °C |
| Molar Mass | 101.1 g/mol |
| Density | 2.109 g/cm3 |
| Crystal structure | Cubic crystal shape |
More Links
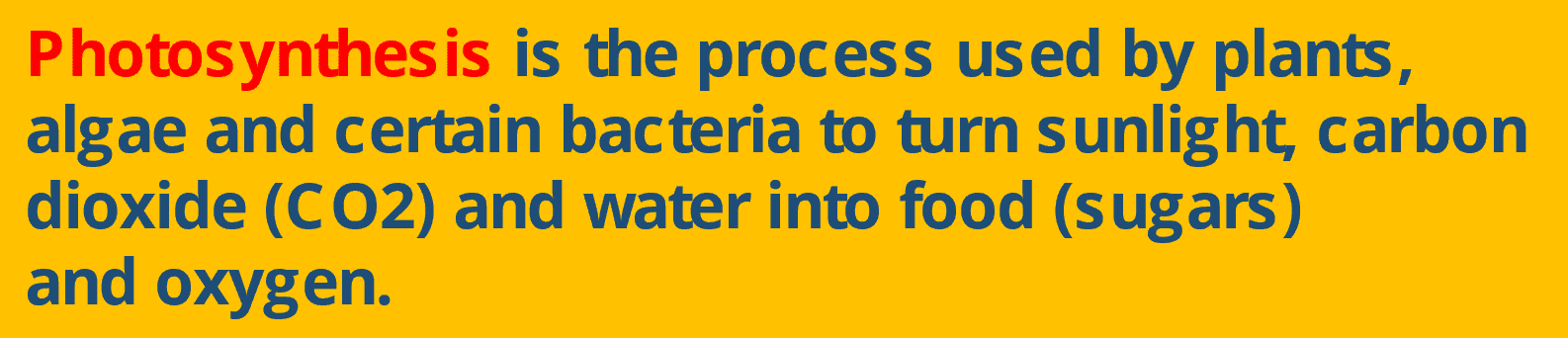
Thermal Energy Equation- Simple Overview
Combustion Reactions| Introduction, Reaction, & Facts
Conduction in Physics| Easy Examples
Sodium Phosphate – Formula, Structure, Types, and Uses
Latest posts by Umair Javaid, PhD Student (see all)
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023