The Helmholtz free energy is a thermodynamic potential defined in thermodynamics as the internal energy minus the product of temperature times entropy. It quantifies the “useful” work produced by a closed thermodynamic system at constant volume and pressure.
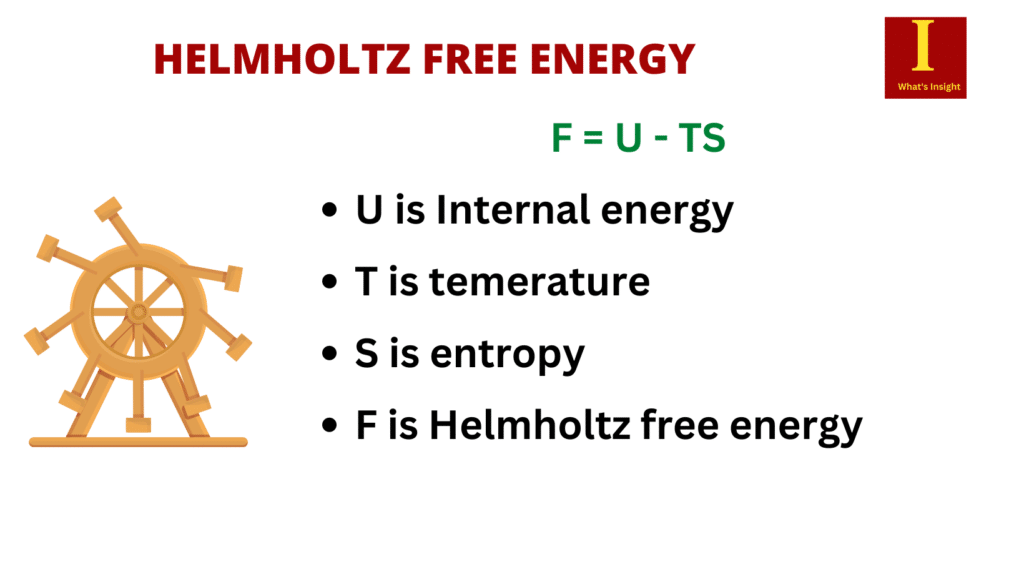
Helmholtz free energy is defined as follows:
F = U – TS
dF = -SdT – pdV
Internal energy, U, has a precise physical meaning: it is the sum of all kinetic and potential energies of all particles in the system. The second term is the amount of spontaneous energy transfer, TS, where S is the system’s final entropy.
Table of Contents
Real Life Examples of Helmholtz Free Energy
Here are some examples of Helmholtz free energy in real-life applications:
- Battery Technology: Helmholtz free energy is used to understand the electrochemical reactions that take place in batteries. By calculating the Helmholtz free energy change of the reaction, scientists can determine the maximum amount of work that can be obtained from the battery.
- Gas Turbines: Helmholtz free energy is used to determine the thermodynamic efficiency of gas turbines. By understanding the Helmholtz free energy of the working fluid, engineers can optimize the turbine design for maximum efficiency.
- Chemical Reactions: Helmholtz free energy is used to determine the equilibrium constant of a chemical reaction. By calculating the Helmholtz free energy change of the reaction, scientists can determine whether the reaction is spontaneous or not.
- Protein Folding: Helmholtz free energy is used to study the folding of proteins. By calculating the Helmholtz free energy change of different conformations, scientists can determine the most stable conformation of a protein.
- Thermal Energy Storage: Helmholtz free energy is used to design thermal energy storage systems. By understanding the Helmholtz free energy change of different storage materials, engineers can optimize the system for maximum energy efficiency.
Step by Step Derivation of Helmholtz Free Energy Equation
here are the steps to derive the formula for Helmholtz free energy:
- Start with the first law of thermodynamics, which states that the change in internal energy (U) of a system is equal to the heat added to the system (Q) minus the work done by the system (W), or U = Q – W.
- Rearrange the equation to get W = Q – U.
- For a system that is undergoing a reversible process at constant volume, the work done by the system is equal to -PdV, where P is the pressure and dV is the change in volume.
- Substitute W = -PdV into the equation from step 2 to get Q = U – PdV.
- From the definition of entropy (S), we know that dS = dQ/T, where T is the temperature.
- Substitute Q = U – PdV from step 4 into dQ = TdS to get dU = TdS – PdV.
- Rearrange the equation to get dU – TdS + PdV = 0.
- This expression is known as the fundamental equation of thermodynamics. By definition, it must be an exact differential, which means that its partial derivatives are independent of the path taken between two states of the system.
- Take the partial derivative of the expression with respect to entropy at constant volume (dV = 0) to get dU/T = dS/T – PdV/T.
- Rearrange the equation to get dU – TdS = -PdV.
- Substitute this expression into the equation from step 4 to get Q = (dU – TdS) + PdV.
- Define the Helmholtz free energy (A) as A = U – TS.
- Take the differential of this expression to get dA = dU – TdS – SdT.
- Substitute dU – TdS from step 10 into the equation from step 13 to get dA = -PdV – SdT.
- This expression gives us the change in Helmholtz free energy for a system undergoing a reversible process at constant temperature and volume. We can also use it to calculate the maximum amount of non-expansion work that can be obtained from the system at constant temperature and volume.
- The final expression for the Helmholtz free energy is A = U – TS, which is derived from the fundamental equation of thermodynamics and the definitions of entropy and work.
Frequently Asked Questions
| No. | Questions | Answers |
| 1 | What is Helmholtz Free Energy? | Helmholtz free energy is a thermodynamic potential that measures the maximum amount of work that a thermodynamic system can perform at constant temperature and volume. It is denoted by the symbol “A” and is defined as A = U – TS, where U is the internal energy of the system, T is the temperature, and S is the entropy. |
| 2 | How is Helmholtz Free Energy related to the spontaneity of a process? | The change in Helmholtz free energy, ΔA, is related to the spontaneity of a process through the equation ΔG = ΔH – TΔS, where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy. If ΔA is negative, the process is spontaneous at constant temperature and volume. |
| 3 | What is the significance of the Helmholtz Free Energy in chemistry and physics? | Helmholtz free energy is a fundamental concept in chemistry and physics as it provides information about the work that can be extracted from a system, as well as the conditions under which a process is spontaneous. It is used to determine the equilibrium state of a system, and it can also be used to calculate the maximum non-expansion work that can be obtained from a system at constant temperature and volume. It is particularly useful in the study of chemical reactions, where it can be used to determine whether a reaction is spontaneous or not, and to calculate the equilibrium constant. |
| 4 | How is the Helmholtz Free Energy related to the partition function? | The Helmholtz free energy is related to the partition function through the equation A = -kTln(Z), where k is the Boltzmann constant, T is the temperature, and Z is the partition function. The partition function is a thermodynamic function that describes the distribution of particles in different energy states, and it can be used to calculate thermodynamic properties such as the Helmholtz free energy, the internal energy, and the entropy. |
| 5 | What is the difference between the Helmholtz Free Energy and the Gibbs Free Energy? | The Helmholtz free energy and the Gibbs free energy are both thermodynamic potentials that provide information about the work that can be extracted from a system. The main difference between them is that the Helmholtz free energy is used to calculate the maximum non-expansion work that can be obtained from a system at constant temperature and volume, while the Gibbs free energy is used to calculate the maximum non-expansion work that can be obtained from a system at constant temperature and pressure. The Gibbs free energy is denoted by the symbol “G” and is defined as G = H – TS, where H is the enthalpy, T is the temperature, and S is the entropy. |
More Links
- BCl3 Lewis Structure in four simple steps - November 1, 2023
- PH3 Lewis Structure in four simple steps - October 8, 2023
- PF3 Lewis structure in four simple steps - September 24, 2023